Better Transfer
Efficient transfer of proteins from gel to membrane.

On This Page |
Transfer Basics | Transfer Methods | Validating Transfer | Transfer Buffers | Membrane Types | Power Conditions & Transfer Time | Tips for Better Transfer | Protocols & Resources |
Sign Up for Western Blotting Tips and Webinar Alerts
Transfer Basics
Protein Transfer on to a Membrane
In western blotting, following electrophoresis is the transfer step. This step consists of moving the proteins from a gel matrix to a synthetic membrane support where it is bound, forming the blot.
Protein Blotting Workflow
Electrophoretic Transfer
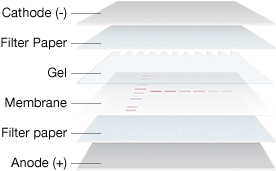
Gel and Membrane Setup for Electrophoretic Transfer
The most common method of transfer in western blotting is electrophoretic transfer, where an electric field is used to elute proteins from gels and transfer them to membranes. During this process, the membrane and gel are placed together, with filter paper between two electrodes. Voltage is applied between the electrodes and proteins migrate to the membrane following the current that is generated by the applied voltage across the electrodes.
The electric field strength (measured in V/cm) that is generated between the electrodes is the driving force for transfer. Both the voltage and the distance between the electrodes then play a major role in governing the rate of elution of the proteins from the gel. Transfer conditions must be optimized for proteins of different molecular weights, e.g., to prevent under-transfer (incomplete transfer of proteins out of the gel) or over-transfer (loss of proteins passing completely through the membrane).
Transfer Methods
There are three main types of transfer apparatus and procedures:
tank transfer systems, semi-dry systems, and rapid-transfer systems.
Tank Transfer Systems

These systems are useful for most routine protein work and for transfers of proteins of all sizes; gels and membranes are submerged under transfer buffer in tanks.
Semi-Dry Systems

Gels and membranes are sandwiched between buffer-wetted filter papers that are in direct contact with flat-plate electrodes. These systems are typically easier to set up than tank systems.
Rapid-Transfer Systems
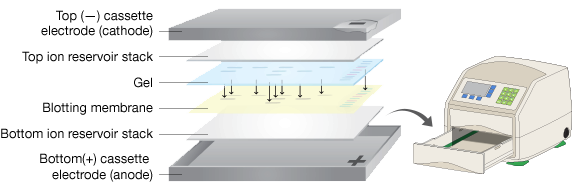
A recent development, these systems utilize specialized apparatus that use proprietary filter papers and specialized buffers to rapidly and efficiently transfer proteins. Filter papers and membranes are pre-wet and packaged in single-use packages, simplifying assembly of the transfer stack.
Comparison of Western Blotting Transfer Systems
|
|
|
||
|---|---|---|---|
|
|
|
||
| Transfer time | 30 min–overnight | 15–60 min | 3–10 min |
| Handling convenience | Manual assembly of transfer components | Manual assembly of transfer components | Prepackaged, presaturated components |
| Transfer parameters | Widest range of power settings and transfer times | Power and transfer time limited due to lack of cooling options | Preinstalled, customizable programs for transfers of most proteins, user-programmable settings for traditional semi-dry techniques |
| Molecular weight range | Broad range | Best for 30–120 kDa | Broad range |
| Temperature control | Cooling with ice pack or refrigerated water recirculator | None | None |
| Buffer requirement | 1–12 L, system-dependent | 250 ml per blot | No additional buffer required |
See Bio-Rad Western Blotting Transfer Systems in Action
-
Trans-Blot Turbo (Rapid)
-
Tank Transfer System
Validating Transfer
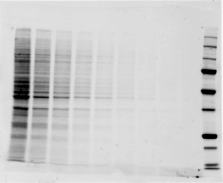
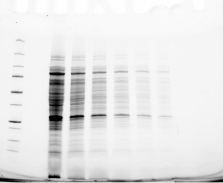
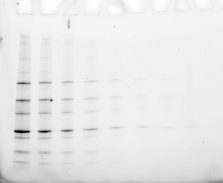
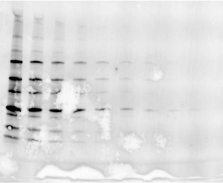
Validating transfer is a critical step in the western blotting process. Validation steps involve using gel and/or membrane stains to visualize the results of your sample transfer prior to moving forward to immunodetection. This visualization allows you to discover if any sample or transfer errors will interfere with your results prior to applying antibody and waiting for incubation, saving you significant time and money if errors have occurred. Stain-free technology enables transfer validation by allowing both the post-transfer gel and membrane images to be collected in under one minute. The membrane is immediately compatible with downstream immunodetection.
Stain-Free Vs. Traditional Western Blotting
-
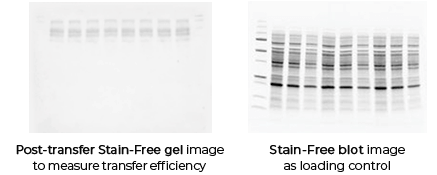
Stain-Free 1min
With stain-free technology you can validate your transfer in a matter of minutes using only a stain-free enabled imager. Gels are still compatible with downstream immunodetection. -
Traditional >1hr
Traditional methods of verifying transfer require staining & destaining which require over an hour to perform.
How transfer validation can help you
The following examples illustrate how the images you record during transfer validation can help you make decisions about proceeding forward with your western blot experiment.
Protein Blotting Workflow
Quick Tips:
Evaluating Western Transfers using Stain-Free Gels and Bio-Rad Imaging System
See how Bio-Rad’s stain-free technology allows you to quickly visualize protein after electrophoresis and verify that all proteins have been transferred out of the gel without the need for additional protein staining.
Transfer Buffers
Transfer buffers contain a conductive, strong buffering agent (for example, Tris, CAPS, or carbonate) in order to maintain the conductivity and pH of the system during transfer. In addition, alcohol (methanol or ethanol) may be included in the transfer buffer to promote binding of proteins to membranes, and SDS may be added to promote elution of proteins from gels.
Compatible Transfer Buffer by Gel Type
| Gel Type | Transfer Buffer | Western Blotting Transfer System |
|---|---|---|
| SDS-PAGE | Towbin with or without SDS, CAPS, carbonate, Bjerrum Schafer-Nielsen | Tank blotting or semi-dry blotting |
| Tris-Tricine | Towbin, CAPS | Tank blotting recommended; needs high-capacity, small pore-size membrane; pH of buffer may be critical |
| Native, non-denaturing | Depends on pH of gel buffer and pI of protein of interest | Tank blotting recommended; temperature regulation may be needed to maintain activity |
| Acid urea | 0.7% acetic acid | Use acid-gel transfer protocol (membrane toward cathode) |
SDS and Alcohol
SDS and alcohol play opposing roles in a transfer. SDS promotes elution of the protein from the gel and in cases where certain proteins are difficult to elute from the gel, SDS may be added to the transfer buffer. However, SDS in the transfer buffer decreases the binding efficiency of protein to nitrocellulose membrane; PVDF membrane can be substituted if desired.
Alcohol, on the other hand, removes the SDS from SDS-protein complexes and improves the binding of protein to nitrocellulose membrane. But alcohol may cause a reduction in the gel pore size, precipitation of some proteins, and some basic proteins to become positively charged or neutral.
Recommended Transfer Buffer by Application
| Application | Recommended Transfer Buffer |
|---|---|
| High-molecular-weight proteins | Towbin with SDS |
| Small proteins and peptides | Towbin, CAPS |
| Basic proteins (pI > 9) in denaturing gels | CAPS, carbonate, Bjerrum Schafer-Nielsen |
| Basic proteins (pI > 9) in native or non-denaturing gels | 0.7% acetic acid |
| Glycoproteins | Towbin with or without SDS, CAPS, carbonate, Bjerrum Schafer-Nielsen nondenaturing gels |
| Proteoglycans | Towbin, Bjerrum Schafer-Nielsen |
-
Transfer Buffer Tips
- Do not use the same batch of transfer buffer more than once, as the buffer will likely lose its capacity to maintain a stable pH during transfer
- Do not dilute transfer buffers; this will also decrease buffering capacity
- Do not adjust the pH of transfer buffers when not indicated, as this increases buffer conductivity, which is manifested by higher initial current output and decreased resistance
Membrane Types
Nitrocellulose and Supported Nitrocellulose
Nitrocellulose membranes are easily wetted in water or transfer buffer, and it is compatible with a wide range of protein detection systems. Unsupported nitrocellulose is innately fragile and are not recommended for stripping and reprobing.
Supported nitrocellulose is an inert support structure with nitrocellulose applied to it. This extra support gives the membrane increased strength and resilience to withstand reprobing and autoclaving (121℃) while maintaining the ease of wetting of nitrocellulose.
Polyvinylidene Difluoride (PVDF)
PVDF membrane has a strong protein binding capacity (about twice that of nitrocellulose) and will not crack nor tear in common handling, so it can withstand repeated stripping and reprobing.
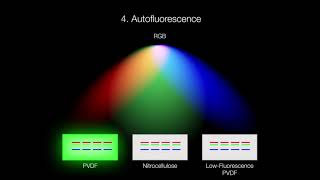
Low-fluorescence formulations of PVDF are available and combine the advantages of PVDF with low autofluorescence across a wide range of excitation and emission wavelengths. This low autofluorescence allows longer exposure times without increasing background fluorescence levels, allowing detection of faint signals.
Pore Size
Membranes are commonly available in two pore sizes:
- 0.45 μm pore size membranes are recommended for most analytical blotting experiments
- 0.2 μm pore size membranes are most suitable for transfer of low-molecular-weight (<15 kDa) proteins that might move through larger membrane pores
Power Conditions and Transfer Time
Factors Affecting Transfer Conditions

Buffer composition
Addition of SDS or changes in ion concentration due to addition of acid or base, changes the resistance of the transfer system and therefore current and results in changes in current and voltage readings as well as transfer efficiency.

Number of gels
Current increases slightly as the number of gels increases. Consider additional cooling or limiting transfer time.

Gel acrylamide percentage
Higher acrylamide percentages slow protein migration out of the gel. Consider extending transfer time.
Proteins move quickly through gels with low acrylamide percentages. Limit transfer time to prevent small proteins from "blowing through" membrane.
Volume of buffer
Current increases when volume increases. Consider additional cooling. Consider additional cooling or limiting transfer time.

Transfer temperature
Current increases when temperature increases.

Protein molecular weight
Large proteins migrate more slowly out of the gel. Consider extending transfer time or increasing voltage.
Conversely, small proteins migrate quickly and may be pushed through the membrane. Consider limiting transfer time or voltage.
-
Western Blot Transfer of High MW Proteins using Trans Blot Turbo Transfer System
For best transfer results, use the highest electric field strength possible within the heat dissipation capabilities of the system. During transfer, the buffer warms as a result of the power dissipated through the system, and its resistance drops. Such heating and changes to resistance may lead to inconsistent field strength and transfer, and may cause the transfer buffer to lose its buffering capacity, or may cause the gel to melt and stick to the membrane.
In general, transfer from thicker or higher percentage gels, or transfer of high-molecular-weight proteins require high electric fields or longer transfer times. Complete protein elution from the gel can be confirmed by post-staining the gel with a total protein stain. Conversely, transfer from thin, low-percentage gels or of proteins of low molecular weight require lower field strengths and shorter transfer times. In some cases, small proteins may “blow through” a membrane. This over-transfer can be detected by placing a second membrane behind the first and staining this membrane with a total protein stain or by imaging the backup membrane using a stain-free imaging system.
Power Settings and Transfer Time by Gel Type
| SDS-PAGE Gels (Towbin Buffer) | ||
|---|---|---|
| Standard (Overnight) | High-Intensity | |
| Trans-Blot Cell plate electrodes |
10 V/100 mA, 16 hr | 50–100 V/700–1,600 mA, 30–60 min |
| Trans-Blot Cell wire electrodes |
30 V/100 mA, 16 hr | 100–200 V/300–800 mA, 30 min–4 hr |
| Trans-Blot Plus Cell | 30 V/0.5 A, 16 hr | 100 V/1,500 mA, 60 min |
| Mini Trans-Blot Cell | 30 V/90 mA, 16 hr | 100 V/350 mA, 60 min |
| Criterion Blotter plate electrodes |
10 V/30–40 mA, 16 hr | 100 V/750–1,000 mA, 30 min |
| Criterion Blotter wire electrodes |
10 V/50–80 mA, 16 hr | 100 V/380–500 mA, 60 min |
| Trans-Blot SD Cell | N/A | Mini gels: 10–15 V/5.5 mA/cm2, 10–30 min Large gels: 15–25 V/3 mA/cm2, 30–60 min |
| Trans-Blot Turbo System | N/A | Mini gels: 25 V/1,300 mA, 7 min Midi gels: 25 V/2,500 mA, 7 min |
Constant Voltage Transfers
If the voltage is held constant throughout a transfer, field strength remains constant, providing the most efficient transfer possible for tank blotting methods. However, the current in tank transfer systems increases as the resistance drops due to heating; in most semi-dry systems, current drops as a result of buffer depletion. Therefore, the overall power increases during transfer, and there is an increased risk of heating. Use of the cooling elements available with the various tank blotting systems helps prevent problems with heating.
Constant Current Transfers
If the current is held constant during a run, a decrease in resistance results in a decrease in voltage and power over time and therefore lower risk of heating. However, proteins are transferred more slowly due to decreased field strength.
Tips for Better Transfers

Remove Bubbles.
Uniform protein transfer requires complete contact between the components of the transfer stack, especially between the gel and membrane. Air bubbles cause blank spots on the membrane and proteins to “flow” around the air bubble, so use a gel roller and roll those air bubbles out! Tip: Gradient gels are best rolled up-down, rather than side-to-side. Side-to-side rolling causes gradient gels to warp and wrinkle.
Equilibrate before Transfer.
When performing traditional wet tank transfers and traditional semi-dry transfers, optimal results are obtained when the electrophoresis buffer saturating the gel is replaced with transfer buffer. Contaminating buffer salts increase the conductivity of the transfer buffer resulting in excess heat during the transfer. Rinse gels briefly in water then equilibrate the gel for 15 minutes and the membrane for at least 5 minutes in transfer buffer. Note that gel equilibration is not recommended for some rapid transfer systems, follow the manufacturer’s directions.

Don't Allow Membranes to Dry Out.
Transfer requires that the membrane stays wet in transfer buffer both before and after transfer. Heat generated during a transfer can dry out membranes once removed from the transfer stack. This is especially important with PVDF membranes since they are hydrophobic. Work quickly, and have a dish of buffer ready to immerse your membrane into in order to avoid drying the membrane. If they do dry, nitrocellulose can simply be re-wet in buffer while PVDF membranes will require re-activation in alcohol.
Western Blot Transfer Protocols and Resources

Find the right products for you using the free Western Blot Selector Tool
Start Tool
Western Blotting Protocol Library
Filter by your laboratory set-up and reagents to get a custom western blotting protocol that best fits your needs.

Stain-Free Western Blotting Guide
Find out how Stain-Free technology can revolutionize your western blotting.

Better Western Blotting Guide
Tips, Techniques, and Technologies from the Western Blotting Experts at Bio-Rad Laboratories.

Protein Blotting Guide
(PDF 7.9 MB)Details on blotting technology, methods, products, tips, techniques, and troubleshooting guidelines.

Western Blot Doctor
Our self-help troubleshooting guide covers solutions to many common and not-so-common western blotting issues and helps your blots look their best.
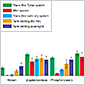
Transfer of High-Molecular–Weight Proteins
Learn more about the relative transfer efficiencies of tank, semi-dry, and rapid blotting systems for western blotting with large proteins.

Membrane Selection Guide
Choose the right blotting membrane material, porosity, and form factor for your western blotting experiments.

Fundamentals of Western Blotting Course #2: Gel Electrophoresis and Transfer

Request your Free Electrophoresis & Western Blotting Layout Post-It
This simple tool allows users to keep track of their Western Blotting experiment from sample preparation to imaging.
Request Yours
Get Your Free Protein Standard Temporary Tattoo
You too can sport a Precision Plus Protein Kaleidoscope standard tatto temporarily.
Get Yours Now
Western Blot University
Courses designed to make you a western blotting expert.
Enroll Today