
Raise your QC skills to the next level with P.A.C.E.-approved program supporting QC lab professionals.

Boost your knowledge and earn C.E. credit with insightful webinars from renowned industry experts and thought leaders across the globe.

Elevate your skills by unlocking valuable educational resources that can help you work smarter, improve quality, and grow professionally.
Low/Mid Volume Routine Laboratories
Maintain Dependable Lab Testing Practices
Maintaining good clinical lab practice is essential for small to medium-sized routine clinical labs to provide consistent, high-quality patient test results. Successful lab operations include basic quality control management, documentation of QC test results, and meeting lab accreditation requirements. For even greater assurance of test quality labs can compare QC results with other labs using QC data management software with peer groups.
As your lab aspires to advance its quality control practices and knowledge, Bio-Rad supports your journey with educational resources and thought leadership.
Leader in QC Education

Full Spectrum of Training Around QC Solutions
Be inspired to learn and do more with our training and educational resources. By offering everything from basic training to advanced theory, our aim is a continuously higher standard in lab QC.
High-Throughput Complex Laboratories
Facilitate Quality Patient Care Through Reliable Results
In a fast-paced high-throughput lab, conflicting priorities impact day-to-day operations, but patient care is always top priority. Patient test results must be reliable and timely as they impact both patient care and a lab’s reputation. Using optimized QC practices and products that incorporate risk-based quality goals and real-time insights improve lab workflow efficiencies and increase confidence in reported patient test results.
Your lab can elevate its quality control practices and set itself apart from others with resources available through Bio-Rad.
Improve Workflow & Manage Risk

Streamlined, Automated Workflow
Our barcoded QC solution allows labs to eliminate manual pour-off tasks and manual data entry, helping to automate and streamline daily QC workflow. Simply load InteliQ quality control onto your instrument and go.
Industry Leader in Independent QC
Leader Stats
300+
Independent controls
500+
Comprehensive analytes
53K+
Instruments participating
65M+
QC results processed monthly
Laboratory Standards
“The use of third-party IQC material should be considered either as an alternative to, or in addition to, control materials supplied by the reagent or instrument manufacturer.”
ISO15189: 2022
"...quality control materials should be different from the calibrator materials to ensure that the QC procedure provides an independent assessment of the measurement procedure’s performance..."
CLSI C24 4th ed., 5.2.5 Relation to Calibrators Clinical and Laboratory Standards Institute (CLSI)
“The laboratory shall design internal quality control systems that verify the attainment of the internal quality of results.”
ISO 15189: 2012(E), Subclause 5.6.2.1.
Customer Testimonials
“The documentation of Bio-Rad Controls using the Unity QC Program has been well accepted by CAP inspectors. Worry-free QC.”
Laboratory Director
Health Center
“I have been extremely happy with Bio-Rad Laboratories’ controls and Unity program for 10+ years.”
Laboratory Professional
Hospital Laboratory
"To compare our data with a substantial peer group ensures that we are turning out reliable patient data."
Laboratory Supervisor
School of Medicine
Quality Management

Laboratory Quality Management Systems
Bio-Rad offers quality control products and services to help every aspect of the quality management testing process, including pre-analytical, analytical, and post-analytical processes to meet regulatory requirements.

QC Data Management Solutions
Compare your data to labs worldwide and develop efficient and reliable data management with Bio-Rad’s Unity QC software.

Quality Control
Bio-Rad’s independent quality control products and services truly power exceptional labs. Reliable, different, and unbiased.
Frequently Asked Questions
Featured FAQ's
What is an independent quality control?
The term “independent" quality control is used to describe a control material that:
- Provides an unbiased, independent performance assessment for an analytical process
- Is not usually formulated to work with any specific test system
- Is manufactured independently of instrument, calibrators, or reagents
Why should I use an independent quality control?
Many instrument manufacturers provide both calibrators and control materials for use on their test systems. These controls are often manufactured from the same materials as the calibrators. Consequently, the control may mimic the calibrator, making it less sensitive to changes in test performance. This may lead to patient test results with analytical error that could be medically important. In addition, independent assayed controls usually include values for test systems from multiple manufacturers.
What are some other benefits of using independent quality controls?
Laboratory using an instrument manufacturer or in-kit control may receive a different control lot with each new reagent lot. A control with a long shelf life allows for long-term QC monitoring across different reagent lots and saves time and money due to fewer lot crossovers.
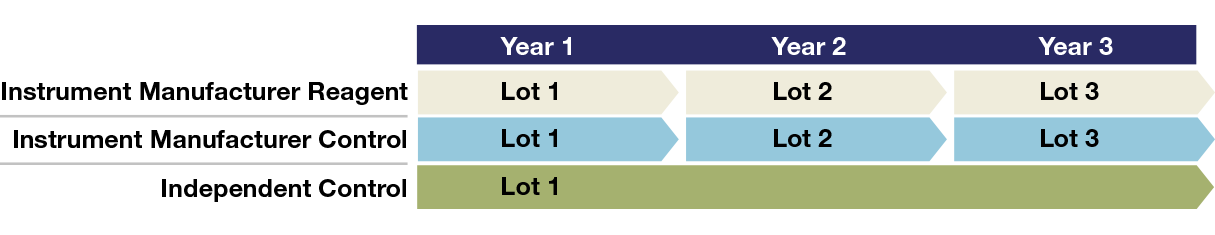
Are there any specific guidelines or recommendations?
Yes. There are several professional organizations that specifically recommend using independent quality controls.
What is the difference between a calibrator and a control?
Calibrators and controls are not the same. They are designed to be used for two completely different purposes.
- A calibrator is a material or in vitro medical device with known quantitative/qualitative characteristics (concentration, activity, intensity, reactivity) that is used to calibrate, graduate, or adjust a measurement procedure
- A control is used to monitor test performance within desired limits
Should a calibrator also be used as a control?
No. If the same calibrator is also used as a control, then the control will closely mimic the calibrator. In this situation, the control may not be able to detect shifts in values that could be caused by a degrading calibrator.
Can I use Bio-Rad controls with Bio-Rad instruments and methods?
Yes. Bio-Rad is the worldwide leader in independent quality controls for the clinical laboratory. Our controls are manufactured independently of the calibrators and reagents and are not optimized to work with any specific instrument or method. Bio-Rad controls provide an unbiased, independent assessment regardless of the instrument or reagent manufacturer. For additional questions, please contact your local representative.







