Read about the current state of the industry and challenges associated with potency assays throughout the manufacturing process.

Learn how advanced nucleic acid quantification technologies can support accurate, streamlined potency assays.

Explore the analytical tools and importance of selecting appropriate methods for the development of potency assays for cell and gene therapy products.
The Importance of Potency Assays for Cell and Gene Therapies During Product Development
Cell and Gene Therapies in Brief
Medical treatments have advanced to using cells and genetic materials to treat or prevent diseases. They work by modifying or replacing the patient's own cells with healthy ones, offering a potential cure for previously untreatable diseases.
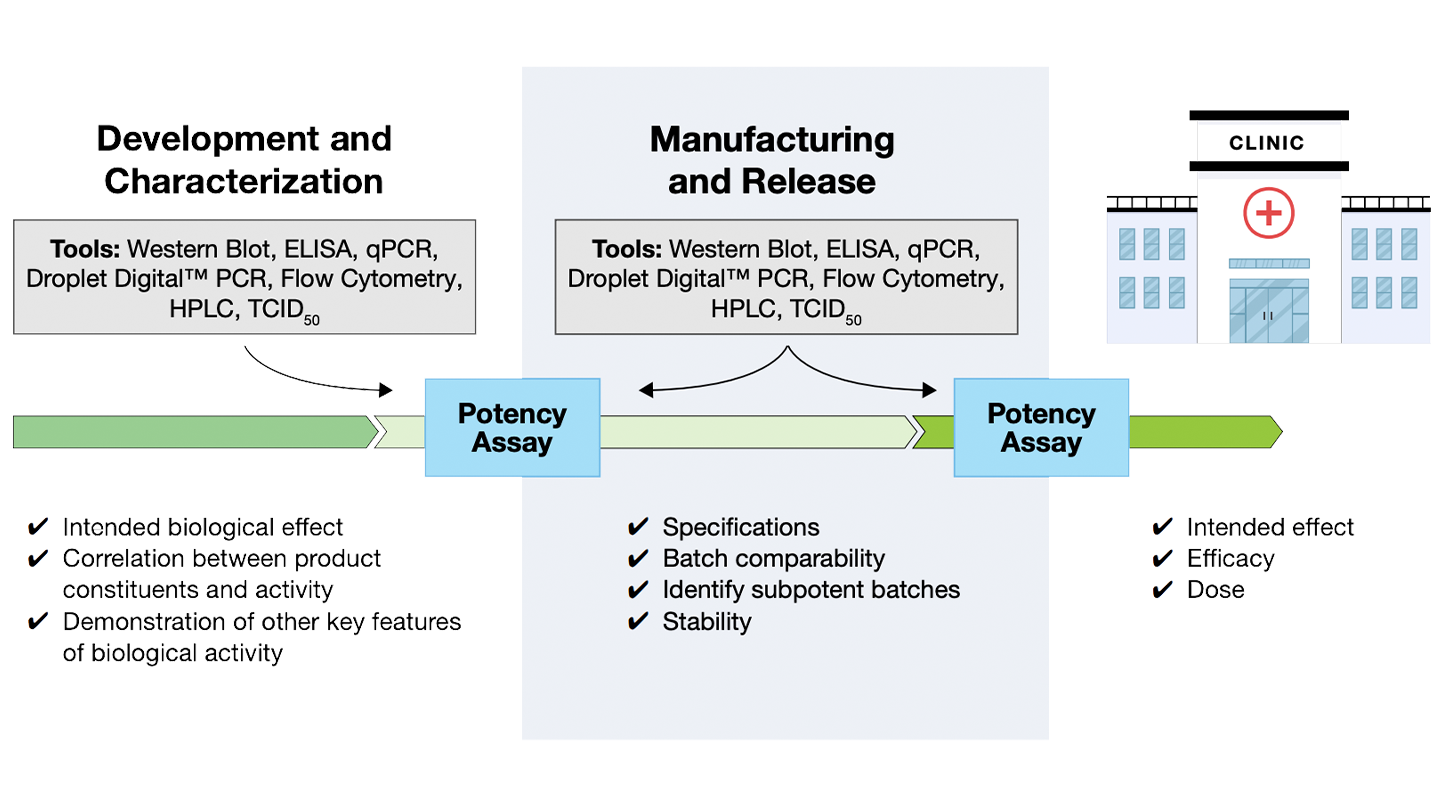
Why is it Important to Measure Cell Potency?
As with all pharmaceuticals, cell and gene therapy (CGT) products must meet rigorous safety guidelines. Potency assays are required to show the intended biological effect or therapeutic activity by using a combination of complimentary assays to assess the mechanism of action.
Cell Therapy Development
Cell Therapy Development Solutions
Develop well-characterized therapeutics out of living cells.
Gene Therapy Development
Improved Gene Therapy Development
Develop better gene therapies with added accuracy, efficiency, and consistency from viral vector characterization to potency assay development.
Analytical Tools for Potency Assays
Potency Assay Development
Potency assays for CGT biotherapeutics must reflect both expression and activity. All this can be achieved using a variety of analytical methods.
Resources
Featured Categories

Find Your Ideal ddPCR Solution with Our Selector Tool
No matter what your research focus is, our interactive tool helps you identify the right Droplet Digital PCR (ddPCR) solution for your specific application.

dPCR or qPCR? Use Our Selector Tool to Guide Your Choice
Quickly determine whether digital PCR or qPCR is right for your experiment. Use our interactive tool to get tailored recommendations.

Meet the Next Evolution of ddPCR Solutions
Sensitivity. Simplicity. Performance. Bio-Rad offers the most comprehensive line of digital PCR products. With the addition of four new instruments, our portfolio sets a new benchmark in providing advanced Droplet Digital™ PCR (ddPCR™) solutions, world-class expertise, and over 12,000 publications to support your research.