
On This Page |
Basic Lab Technique |
Select Your Assay Chemistry |
Optimizing RT-qPCR |
DNA Binding Dyes |
Multiplex Fluorescent Chemistries |
Design and Optimize Assays |
Related Products |
Resources |
The sections below are chosen from the Bio-Rad Real-Time PCR guide and offer recommendations and tips for qPCR assay design, as well as lists of useful reagents and free online software programs that can aid primer and assay design.
Ensuring that a qPCR assay is quantitative, accurate, and reproducible requires thorough preparation in terms of assay design, setup, and optimization.
The Phases of a PCR Reaction
Real-time PCR is a technique in molecular biology in which a target molecule of DNA is amplified by repeat cycles of PCR, and this amplification is monitored after each cycle of PCR, in real time, and not just at its end-point, as occurs in conventional PCR (or end-point PCR). Amplification and quantification occur simultaneously using specialized thermal cyclers equipped with fluorescence detectors.
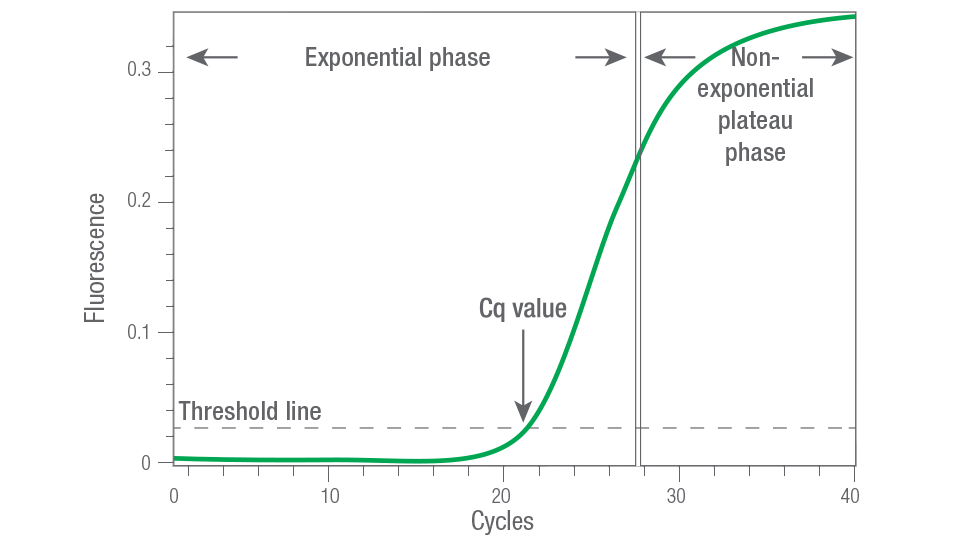
Figure 1. Exponential and plateau phases of the PCR reaction. Real-time-qPCR (or qPCR) measures the PCR reaction at the exponential phase of PCR amplification, before it reaches the plateau phase. RT-qPCR is more quantitative because it can detect small differences in expression levels between samples. qPCR can also provide the cycle quantitation, or Cq, which is the cycle where the reaction has a fluorescence intensity above background.
Assay design and optimization are the keys for reliable quantitation during gene expression using quantitative PCR (qPCR) assays. Ensuring that a qPCR assay is quantitative, accurate, and reproducible requires thorough preparation in terms of assay design, setup, and optimization. Poor qPCR assay design — unsuitable reaction conditions (e.g., choice of master mix, primer or probe Tm), the presence of inhibitors or underlying single-nucleotide polymorphisms (SNPs) — can lead to suboptimal amplification and extensive troubleshooting.
The qPCR process involves fluorescent reagents, which allow researchers to observe PCR product accumulation during the entire amplification process, eliminating the need to run a gel, shortening process time and reducing the chance of contamination. Amplification and quantification occur simultaneously using specialized thermal cyclers equipped with fluorescence detectors. The sections below offer recommendations and tips for qPCR assay design, as well as lists of useful reagents and free online software programs that can be used to aid primer and assay design.
Basic Laboratory Technique for qPCR Assays
Proper laboratory technique is an important yet often overlooked step toward successful real-time PCR. For optimal results, you should minimize the potential for cross-contamination between samples and prevent carryover of nucleic acids from one experiment to the next. The following precautions can help you avoid contamination problems:
- Wipe down workstations with dilute solutions of bleach or with commercially available decontamination solutions
- Prepare samples in a designated clean room, hood, or benchtop workstation equipped with a UV lamp. Ideally, this should be located in a different area from the thermal cycler. Take care to avoid contaminating the area with plasmids or amplicons; never bring postamplification products into the designated clean area
- Change gloves frequently during sample preparation and reaction setup
- Use shaftguard pipets and aerosol-barrier pipet tips
- Clean pipets frequently with a dilute solution of bleach
- Use PCR-grade water and reagents dedicated for PCR use only
- Use screw-capped tubes for dilutions and reaction setup
- In addition to taking the above precautions, you should always prepare a no-template control for your PCR assays to verify that no contamination has occurred. Furthermore, use a hot-start polymerase to prevent indiscriminate amplification before the reaction begins.
How to Select Your Assay Chemistry for qPCR
The chemistry you select for your qPCR assay depends on your application, whether you’re performing singleplex or multiplex reactions, and cost considerations. In general, for low-throughput, singleplex experiments, DNA-binding dyes may be preferable because these assays are easier to design, are faster to set up, and are initially more cost-effective. For high-throughput experiments, a fluorescent primer- or probe-based assay (singleplex or multiplex) may be more desirable because of the lower average cost per sample and the reduction in assay time from multiplex capability.
Multiplex assays require the use of a fluorescent primer- or probe-based chemistry, because the lack of specificity of DNA-binding dyes makes them incompatible with quantitative multiplex assays. With multiplexing, you can amplify and quantify as many as five targets in a single tube, depending on the features of your real-time PCR instrument. Multiplexing offers these advantages over singleplex assays: lower requirement of starting template, ability to include internal assay control, higher throughput for time and cost savings, and lower sample handling.
Guidelines for Optimizing RT-qPCR
A successful qPCR assay requires efficient and specific amplification of the product, and using PCR primers specifically designed and validated for qPCR assays is essential. The choice of target sequence and primer design is important because both can affect amplification efficiency and specificity and thus the accuracy of qPCR assays.
The following steps will help ensure a successful qPCR reaction:
- Verify RNA quality (purity) and yield
- Confirm the primers chosen span long introns
- Optimize the reverse transcription reaction
- Use a high-quality reverse transcription kit to enable a representative cDNA copy of the mRNA
- Verify your negative controls (these are the no-reverse transcription controls)
Be sure to view the qPCR Assay Design and Optimization page for specific information and tips on designing qPCR assay primers and probes, choosing a target sequence, multiplexing, selecting reporters and quenchers, and validating and optimizing qPCR assays.
Reverse Transcriptase Products
Ultimately, a good reverse transcription (RT) kit is useful to produce a complete and representative cDNA copy of the mRNA.
The hallmarks of a good RT kit include: 1) a mixture of random hexamers and oligo(dT)s to assure complete coverage of the transcriptome; 2) RNase H to digest the RNA while the cDNA is synthesized, which minimizes bias in cDNA production preventing the Cq values from being skewed to lower and variable values that would be unrepresentative of the true target amount in each sample; 3) an RNase inhibitor to prevent degradation of the RNA prior to RT; 4) a highly robust RT enzyme that permits RT of the widely ranging concentration of transcripts populating each sample. iScript Reverse Transcription Reagents from Bio‑Rad contain a blended mixture of each of these components to assure cDNA that closely reflects the transcriptome.
Reliance One-Step Multiplex Supermix from Bio-Rad offers a good example of a high-performance RT enzyme combined with an advanced reaction buffer which is designed for one-step RT-qPCR. The supermix format delivers reproducible performance for multiplexed RT-qPCR, and can be used with challenging RNA templates, difficult targets or in the presence of PCR inhibitors.
DNA Binding Dyes — for Singleplex Assays Only
The most commonly used DNA-binding dye for real-time PCR is SYBR® Green I, which binds nonspecifically to double-stranded DNA (dsDNA). In this dye, the fluorescence dramatically increases when the dye molecules bind to dsDNA.
The advantages of using dsDNA-binding dyes include simple assay design (only two primers are needed; probe design is not necessary), ability to test multiple genes quickly without designing multiple probes, e.g., for validation of gene expression data from many genes in a microarray experiment, lower initial cost (probes cost more), and the ability to perform a melt-curve analysis to check the specificity of the amplification reaction. The major drawback of DNA-binding dyes is their lack of specificity, that is, DNA-binding dyes bind to any dsDNA and can increase the presence of non-specific products. In addition, they cannot be used for multiplex reactions. Melt-curve analysis can be used to identify different reaction products, including nonspecific products, in the SYBR Green example shown below.
Melt curve analysis can be used to identify different PCR products, including nonspecific products and primer-dimers when the fluorescence of the reporter chemistry depends on the presence of dsDNA, as with SYBR Green dye. This property is valuable because the presence of secondary nonspecific products and primer-dimers can severely reduce the amplification efficiency and, ultimately, the accuracy of the qPCR assay. Primer-dimers can also limit the dynamic range of the desired standard curve due to competition for reaction components during amplification.
After completion of the amplification reaction, a melt curve is generated by increasing the temperature in small increments and monitoring the fluorescence signal at each step. As the dsDNA in the reaction is denatured, the fluorescence decreases rapidly and significantly. A plot of the negative first derivative of the fluorescence versus temperature, –d(RFU)/dT vs. T, the rate of change of fluorescence intensity) displays distinct peaks corresponding to the Tm of each product (Figure 2).
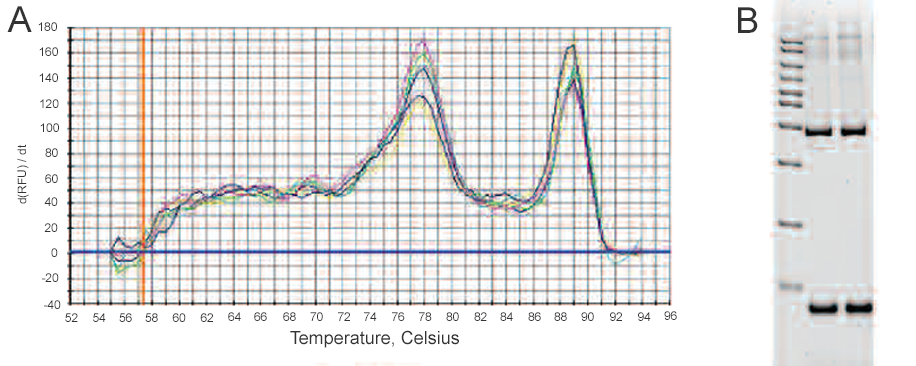
Figure 2. Melt curve analysis of products from a SYBR Green Assay. A, the negative first derivative of the change in fluorescence plotted as a function of temperature. The two peaks indicate the presence of two PCR products; B, gel analysis of the qPCR products. Lane 1, AmpliSize® 50–20,000 bp molecular ruler; lanes 2 and 3, two replicates of qPCR assay products from the reaction shown in A. The two separate bands in the gel confirm the presence of two PCR products.
The melt peak distinguishes specific products from other products that melt at different temperatures, such as primer-dimers. Because of their small size, primer-dimers usually melt at lower temperatures than the desired product, whereas nonspecific amplification can result in PCR products that melt at temperatures above or below that of the desired product. In the melt curve above, the peak at 89°C represents the target qPCR assay product and corresponds to the upper band in lanes 2 and 3 on the gel. The peak at 78°C represents the nonspecific product and corresponds to the lower band in lanes 2 and 3 on the gel.
Fluorescent Chemistries — for Multiplex Assays
Many fluorescent primer- and probe-based chemistries have been devised and are available from different commercial vendors. The most commonly used probe-based chemistries, TaqMan probes and molecular beacons, are discussed in detail below. Brief descriptions of other fluorescent primer- and probe-based chemistries, including Eclipse probes and Amplifluor, Scorpions, LUX, and BD QZyme primers, are also presented below.
In real-time PCR, fluorescent primers and probes offer two main advantages over DNA-binding dyes. First, they specifically detect the target sequence so nonspecific products do not affect the accuracy of quantification. Second, they allow multiplex reactions to be performed.
TaqMan Assays
TaqMan assays employ a sequence-specific, fluorescently labeled oligonucleotide probe called the TaqMan probe, in addition to the sequence-specific primers. Figure 3 illustrates how TaqMan assays work. Also known as the 5'-nuclease assay, the TaqMan assay exploits the 5'-exonuclease activity of certain thermostable polymerases, such as Taq or Tth. The probe contains a fluorescent reporter at the 5' end and a quencher at the 3' end. When intact, the fluorescence of the reporter is quenched due to its proximity to the quencher. During the combined annealing/extension step of the amplification reaction, the probe hybridizes to the target and the dsDNA-specific 5'—>3' exonuclease activity of Taq or Tth cleaves off the reporter. As a result, the reporter is separated from the quencher, and the resulting fluorescence signal is proportional to the amount of amplified product in the sample. One commonly used fluorescent reporter-quencher pair is fluorescein (FAM, which emits green fluorescence) and Black Hole Quencher 1.
The main advantages of using TaqMan probes include high specificity, a high signal-to-noise ratio, and the ability to perform multiplex reactions. The disadvantages are that the initial cost of the probe may be high and the assay design may not be trivial.
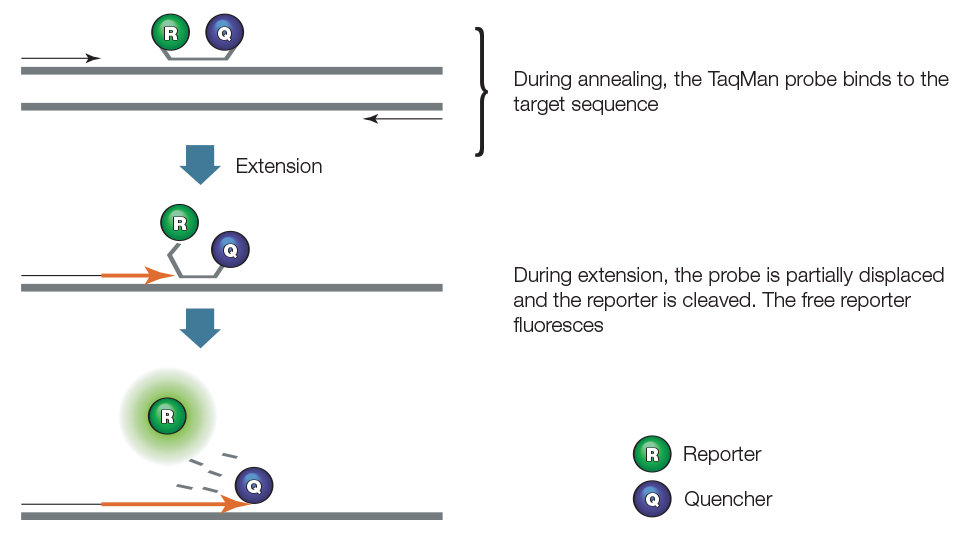
Figure 3. TaqMan assay.
Molecular Beacons
In addition to two sequence-specific primers, molecular beacon assays employ a sequence-specific, fluorescently labeled oligonucleotide probe called a molecular beacon, which is a dye-labeled oligonucleotide (25–40 nt) that forms a hairpin structure with a stem and a loop (Figure 4). A fluorescent reporter is attached to the 5' end of the molecular beacon and a quencher is attached to the 3' end. The loop is designed to hybridize specifically to a 15–30 nucleotide section of the target sequence. On either side of the loop are 5–6 nt that are complementary to each other, and form a stem structure that serves to bring the reporter and quencher together. In the hairpin structure, no fluorescence is detected from the reporter due to its physical proximity to the quencher. During the annealing step of the amplification reaction, the molecular beacon binds to its target sequence, separating the reporter and quencher such that the reporter is no longer quenched. Unlike TaqMan assays, molecular beacons are displaced but not destroyed during amplification, because a DNA polymerase lacking the 5' exonuclease activity is used. The amount of fluorescence emitted by the reporter on the molecular beacon is proportional to the amount of target in the reaction.
Molecular beacons have some advantages over other chemistries. They are highly specific, can be used for multiplexing, and if the target sequence does not match the beacon sequence exactly, hybridization and fluorescence will not occur — a desirable quality for allelic discrimination experiments.
The main disadvantage of using molecular beacons lies in their design. The stem of the hairpin must be strong enough that the molecule will not spontaneously fold into non-hairpin conformations that result in unintended fluorescence. At the same time, the stem of the hairpin must not be too strong, or the beacon may not properly hybridize to the target.
Figure 4. Molecular beacons.
Other Chemistries
Hybridization probe assays employ two sequence-specific oligonucleotide probes, in addition to two sequence-specific primers. The two probes are designed to bind to adjacent sequences in the target, as illustrated in Figure 5. The first probe carries a donor dye at its 3' end, while the second carries an acceptor dye at its 5' end. The donor and the acceptor dyes are selected such that the emission spectrum of the donor dye overlaps significantly with the excitation spectrum of the acceptor dye, while the emission spectrum of the donor dye is spectrally separated from the emission spectrum of the acceptor dye. Excitation is performed at a wavelength specific to the donor dye, and the reaction is monitored at the emission wavelength of the acceptor dye. During the annealing step of PCR, the probes hybridize to their target sequences in a head-to-tail arrangement. This brings the fluorescent molecules into proximity, allowing fluorescence resonance energy transfer from donor to acceptor. The increasing amount of acceptor fluorescence is proportional to the amount of amplicon present.
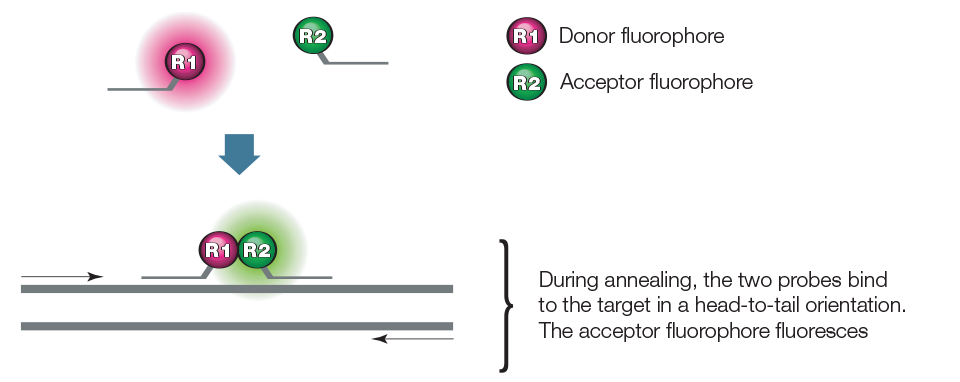
Figure 5. Hybridization probe.
qPCR assays using an Eclipse probe employ two primers and a sequence-specific oligonucleotide probe, as illustrated in Figure 6. The probe is complementary to a sequence within the amplicon and contains a fluorescent reporter at the 3' end, a quencher at the 5' end, and a minor groove binder. The unhybridized probe adopts a conformation that brings the reporter and quencher together, quenching the reporter. During the annealing step of PCR, the probe hybridizes to the target with the help of the minor groove binder. The probe thus becomes linearized, separating the reporter and quencher and allowing the reporter to fluoresce. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
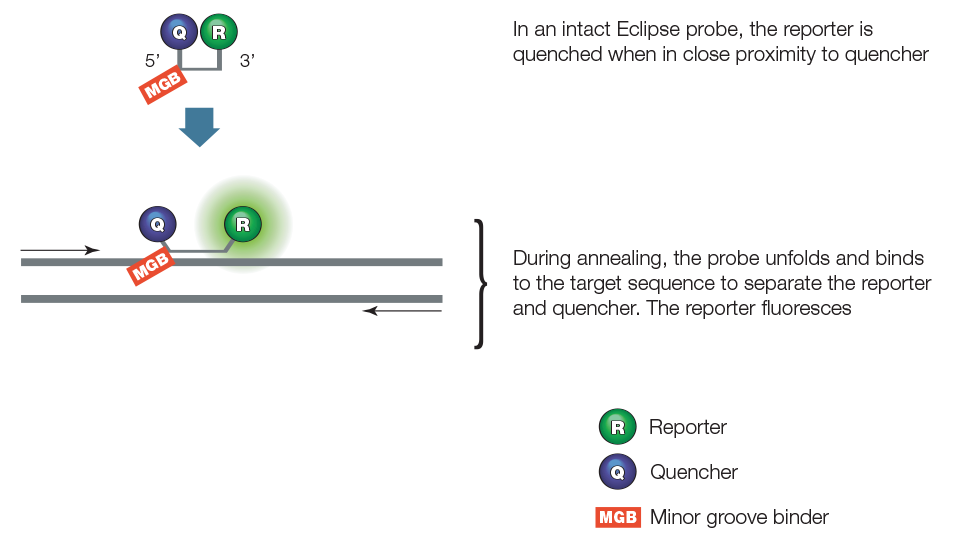
Figure 6. Eclipse probes.
qPCR assays using Amplifluor chemistry employ two target-specific primers and one universal primer called the UniPrimer. The first target-specific primer contains a 5' extension sequence called the Z-sequence that is also found at the 3' end of the UniPrimer. As shown in Figure 7, the UniPrimer forms a hairpin structure. A fluorescent reporter and a quencher are attached at the 5' and the 3' ends of the stem structure, respectively. In the hairpin conformation, the reporter fluorescence is quenched due to its proximity to the quencher. During the first amplification cycle, the first target-specific primer (with the Z-sequence) hybridizes to the template and is extended. During the second amplification cycle, the second target-specific primer is used to synthesize a new target template that contains a sequence complementary to the Z-sequence. The product from the second amplification cycle can then serve as the template for the UniPrimer. In the third amplification cycle, the extended UniPrimer serves as a template for the next amplification cycle. In the fourth cycle, extension of the template through the hairpin region of the UniPrimer causes the UniPrimer to open up and adopt a linear configuration, which allows the reporter to fluoresce. Exponential amplification using the second target-specific primer and the UniPrimer occurs in subsequent amplification cycles. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
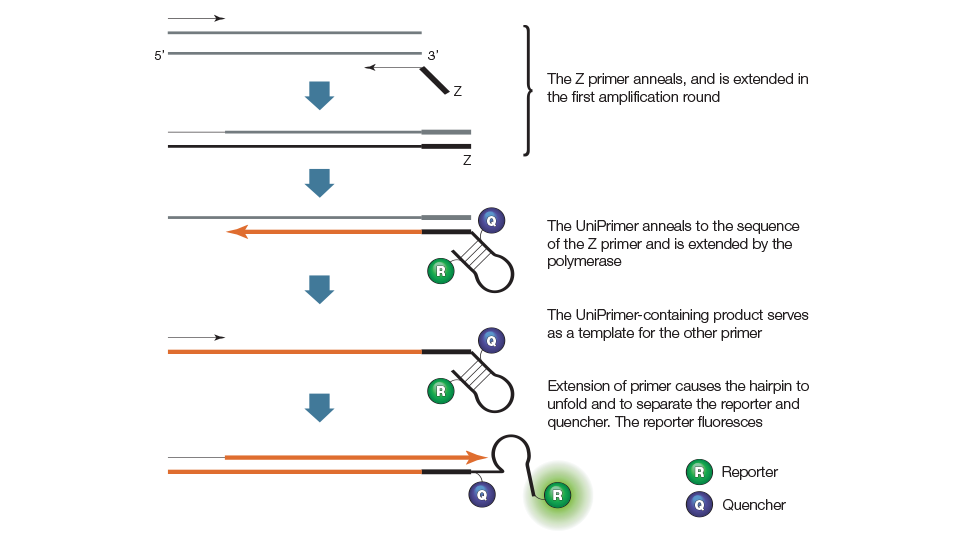
Figure 7. Amplifluor primer.
Scorpions primer assays employ two primers, one of which serves as a probe and contains a stem-loop structure with a 5' fluorescent reporter and 3' quencher, as illustrated in Figure 8. The loop sequence of the Scorpions probe is complementary to an internal portion of the target sequence on the same strand. During the first amplification cycle, the Scorpions primer is extended and the sequence complementary to the loop sequence is generated on the same strand. The Scorpions probe contains a PCR blocker just 3' of the quencher to prevent read-through during the extension of the opposite strand. After subsequent denaturation and annealing, the loop of the Scorpions probe hybridizes to the target sequence by an intramolecular interaction, and the reporter is separated from the quencher. The resulting fluorescent signal is proportional to the amount of amplified product in the sample.
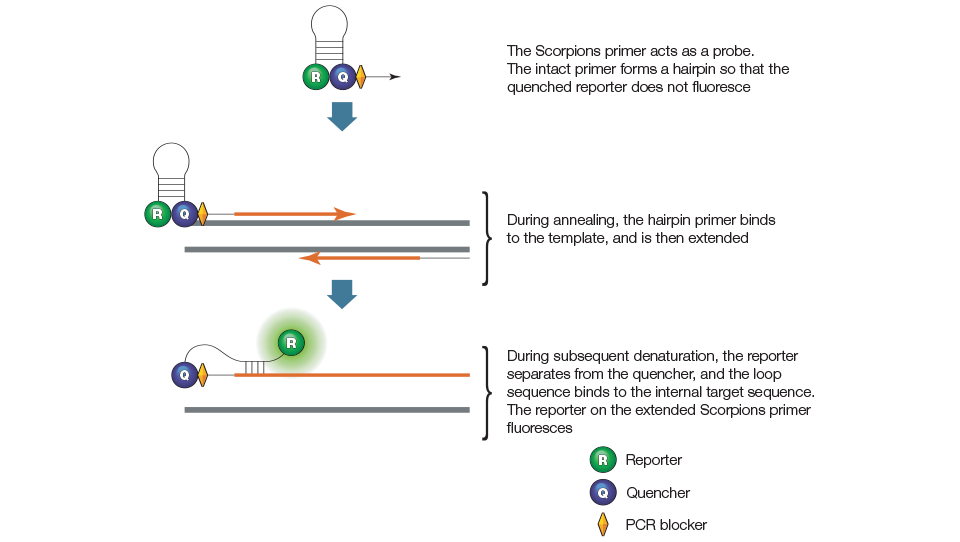
Figure 8. Scorpions primer.
LUX primer assays employ two primers, one of which is a hairpin-shaped primer with a fluorescent reporter attached near the 3' end, as illustrated in Figure 9. In the intact primer, the reporter is quenched by the secondary structure of the hairpin. During amplification, the LUX primer is incorporated into the product and the reporter fluoresces.
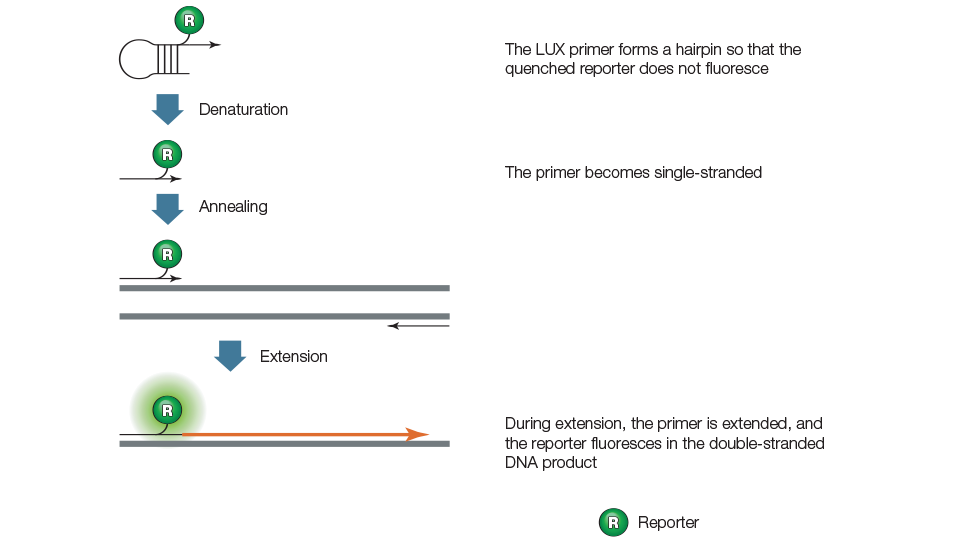
Figure 9. LUX primers.
qPCR assays using BD QZyme primers employ a target-specific zymogene primer, a target-specific reverse primer, and a universal oligonucleotide substrate as shown in Figure 10. The oligonucleotide contains a fluorescent reporter on the 5' end and a quencher on the 3' end. When oligonucleotide substrate is intact, the fluorescence of the reporter is quenched by the quencher due to their proximity. The zymogene primer contains a sequence that encodes a catalytic DNA. During the first amplification cycle, the zymogene primer is extended. In the second cycle, the product of the first cycle is used as the template by the target-specific reverse primer, which is extended to create a new target sequence containing a catalytic DNA region. In the subsequent annealing step, the fluorescently labeled oligonucleotide substrate hybridizes to the catalytic DNA sequence and is cleaved. This cleavage separates the reporter from the quencher, resulting in a fluorescent signal that is proportional to the amount of amplified product in the sample.
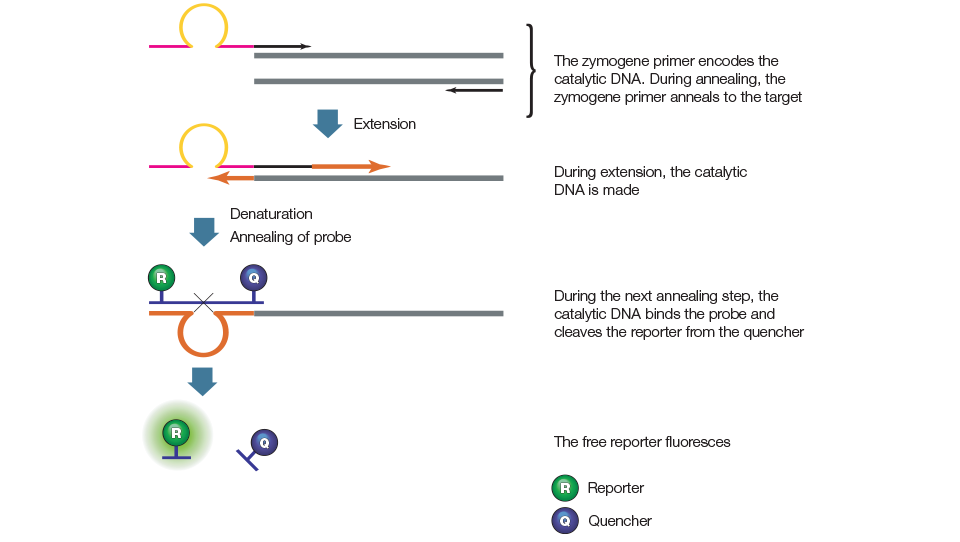
Figure 10. BD QZyme primers.
Design and Optimize Single and Multiplex RT-qPCR Assays
A qPCR assay that meets design and optimization criteria is likely to perform well. These two key steps for developing a qPCR assay are described in the following sections of our PCR Applications Guide:
- Primer and probe design
- Assay validation and optimization
See Our qPCR Optimization Guide »
As noted above, the reverse transcription step in two-step RT-qPCR can be performed using oligo(dT) primers, random primers, a mix of these two, or gene-specific primers. The choice of primers may influence quantification of your target gene. Gene-specific primers give less background priming than oligo(dT) or random primers. If you choose oligo(dT) primers for reverse transcription, you may want to place PCR primers close to the 3' end of the transcript to avoid loss of sensitivity due to truncated messages; this is especially important for longer transcripts.
Oligo(dT) priming should be avoided if you are working with transcripts or species that have short poly(A) tails or lack them altogether. For the 5'-nuclease assay, we found oligo(dT)–primed cDNA to perform very well; however, random-primed cDNA performed equally well or slightly better for most sequences, and much better for some sequences. For most PCR applications, we have found best performance using iScript and iScript Select cDNA synthesis kits, which employ an MMLV RT.
Related Products
-

PrimePCR Validated PCR Assays
Experimentally validated PCR primer and probe assays for gene expression, copy number variation, and mutation detection for real-time PCR and Droplet Digital PCR
-

Reliance Select cDNA Synthesis Kit
The Reliance Select Kit enables researchers to synthesize cDNA with the highest level of sensitivity and robustness by utilizing the unique properties of its novel chimeric reverse transcriptase.
-

iScript cDNA Synthesis Kits
iScript Reverse Transcription Reagents feature an RNase H+ MMLV reverse transcriptase plus an RNase inhibitor for reliable yields.
-

Preamplification Supermix
Ready-to-use supermix for unbiased, target-specific preamplification of cDNA or gDNA using up to 100 qPCR assays
Resources
-

Amplification and Reagents and Plastics Brochure
An overview of PCR reagents and plastics with info on specifications, performance data, and more.
-

End the Cycle of Repeated, Failed Experiments with a Robust Field Guide to qPCR
A snapshot of the critical steps needed to achieve excellent qPCR results.
-

Tips, Tricks & Best Practices for qPCR
Information on the best practices for designing qPCR experiments.
-

PCR Amplification Guide
A guide for RT-PCR on key concepts, experimental designs, analyses, and product recommendations.
-
Validating a Quantitative PCR (qPCR) Experiment to Minimize Error and Maximize Data Quality
A checklist of critical steps needed to achieve excellent results to performing a valid qPCR experiment.
