Cell Therapy Development Overview

On This Page |
How We Help | Where We Help | More on Cell Therapy | Resources |
Helping You Bring the Promise of Cell Therapy Development to Life
Creating standardized treatments from inherently variable material like living cells requires knowing exactly what you are working with, whether you are in discovery, development, or manufacturing. We are here to help.
How We Help
Cell therapies like CAR T cells are already changing lives for those with cancer, even though we are only just beginning to understand how to fully exploit this promising therapeutic approach. One of the biggest challenges is managing the “messiness” and variability of biological systems.1 Creating a standardized, well-characterized therapeutic out of living cells requires accurate, reliable, and often quantitative information about the composition and behavior of your cell therapy.
At Bio-Rad, you will find a range of proven products and services that can deliver actionable insight about the composition and behavior of your cell therapy, whatever stage you are at in the development process. Whether you are working in early discovery or manufacturing and QC, genetically modifying cells or simply selecting, characterizing, and expanding a critical subpopulation, we can help you obtain the data you need to move your cell therapy development to the next step.

Did you know?
As of August 2020, the majority of cell therapy trials found in clinicaltrials.gov by Wang, et al., used T cells (44%), closely followed by stem cells (35%), and then dendritic cells (8%), natural killer cells (7%), microbes (3%), red blood cells (2%), monocytes (2%), and platelets (0.5%).1
Where We Help Advance Your Cell Therapy Development Efforts
-
Antigen Discovery
Discover innovative cell therapy approaches quickly and confidently with automated and quantitative tools for neoantigen discovery, CAR T-cell characterization, CAR construct optimization, and more.
-
Cell Therapy Characterization
Develop critical assays to evaluate potency and batch-to-batch consistency, whether for CAR T-cell characterization or development of another cell therapy approach.
-
In Vivo Bioanalysis
Assess the body’s reaction to your cell therapy by developing in vivo bioanalysis assays to monitor CAR T-cell persistence, exhaustion, and other functional parameters.
-
Manufacturing & QC
Efficiently ensure the safety of your cell therapy manufacturing process, whether you are evaluating transgene copy number or verifying the absence of microbial pathogens.
More on Cell Therapy Development
-

5 Biggest Trends of Gene-Modified Cell Therapy
Learn about key trends in gene-modified cell therapy that have emerged in recent years to shape the future of cellular immunotherapy.
-

Building and Optimizing Complex, High-Throughput Immunophenotyping Panels
Follow best practice guidelines on aspects of immunophenotyping for building, optimizing, and performing complex and high-throughput immunophenotyping panels.
-

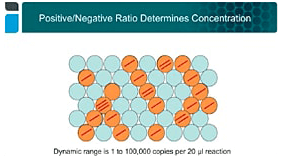
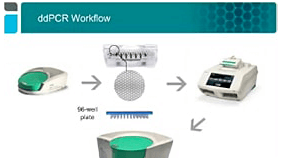
Droplet Digital™ PCR (ddPCR™) Is Well Suited for Quantifying Transgene Copy Number
Discover why CAR T-cell therapy is so exciting, common hurdles in development and manufacturing, and how ddPCR technology is a solution for these issues.
-

Measuring Potency of Cell and Gene Therapy Products
Explore the common challenges, analytical tools, and importance of selecting appropriate assays for the development of potency assays for cell therapy products.
-

Improving Quality Control in CAR T-Cell Manufacturing with Droplet Digital™ PCR
Discover how Droplet Digital™ PCR provides a robust and sensitive method for monitoring and validating CAR T-cell therapies during manufacturing in this article.
-

Understanding Potency in Cell and Gene Therapy Development
Read about the current state of the industry, challenges associated with potency assays, and measuring product potency throughout the manufacturing process.
-
Challenges In Developing CAR T-Cell Therapies
Understand how quality control assays are being implemented in monitoring the development of CAR-T cells using high-throughput technologies in this article.
-

Monitoring CAR T-Cell Therapy Quality with Droplet Digital™ PCR (ddPCR™)
Understand how ddPCR technology provides the sensitivity, precision, and versatility needed to improve quality control for the CAR T-cell manufacturing process.
-

Cell and Gene Therapy Publications Using the ZE5 Cell Analyzer
Get a list of the latest high-profile cell therapy publications using flow cytometry.
-

Transitioning Your Assay from Quantitative PCR to Droplet Digital™ PCR (ddPCR™)
Get guidance on how to transition from qPCR to ddPCR technology and the differences to consider between the two methods based on AAV titering assay study.
Resources
Webinars
-

Can We Trust Western Blots?
Learn how to build confidence in western blotting data and tips for producing high-quality western blots in less time.
-

Expert Coffee Chats — Flow Cytometry Optimizations and Applications
Find flow cytometry topics on gene therapy workflows including considerations for consistency across instruments in multiple labs (46:10) and switching from single tubes to plate-based high-throughput analysis (57:20).
Documents

A Novel CAR T-Cell Therapy Approach Using Fluorescence-Activated Cell Sorting and Stem Cell Transplantation
Find out how researchers at Columbia University Medical Center developed a new approach for CAR T-cell therapy when faced with the lack of unique antigens.

Elucidating Complex Flow Cytometry Studies with the Speed and Sensitivity of the ZE5 Cell Analyzer
Discover how the ZE5 Cell Analyzer obtains biologically relevant data from increasingly complex studies such as exosome analysis and immunophenotyping in a high-throughput manner.

Automation of High-throughput Flow Cytometry with the ZE5 Cell Analyzer
Learn how integrating flow cytometers into automated workcells increases throughput, enables efficient operation, and provides consistent results.

An Optimized Workflow for Generating Anti-CD19 CAR T Cells by mRNA Electroporation
See how a CAR vector can be optimized for T-cell expression with the Gene Pulser Xcell System for transfection, followed by analysis with the TC20 Cell Counter, ZOE Cell Imager, and ZE5 Flow Cytometer.
Receive Updates
Keep up to date with useful tips and online resources to continuously improve your therapeutic antibody development workflows. Sign up to be notified when new resources like publications, protocols, webinars, and how-to videos become available.