
On This Page |
Enabling Discovery | High-Throughput Cell Characterization | CAR Expression Evaluation | Resources |
Keep Efficiency at the Core of Your T-Cell Antigen Discovery Program
Discover innovative cell therapy approaches quickly and confidently with automated and quantitative tools for neoantigen discovery, CAR T-cell characterization, CAR construct optimization, and more.
Enabling Early T-Cell Therapy Discovery
Finding tumor-specific antigens and neoantigens is only one of the many challenges involved in early cell therapy discovery and it is of critical importance when it comes to patient safety. Without highly specific targeting, the destructive power of the CAR T cell could affect non-cancerous cells, leading to off-target toxicity.
Optimizing CAR construct expression to ensure the ideal therapeutic response is another of the early cell therapy discovery challenges.
Efficiently move through these essential yet time-consuming parts of the cell therapy development process with tools that combine ease-of-use with accurate quantitation, multiplex detection, and throughput.

Did you know?
Despite not having access to modern next-generation sequencing tools or even automated Sanger sequencing instruments, De Plaen, et al. were able to identify and sequence an 800 kb DNA fragment containing the first tumor neoantigen in 1988.1
Automation-Ready Flow Cytometry is the Key to Efficient T-Cell Antigen Discovery
Multiplex flow cytometry is an invaluable tool for identifying cell subpopulations and characterizing multiple features on the cell surface. But conventional flow cytometry can be a highly manual process, slowing your T-cell antigen discovery workflows.
With the high-performance, automation-ready ZE5 Cell Analyzer, you can overcome cell characterization analysis bottlenecks and achieve high-throughput screening and multiparameter cell analysis for efficient early cell therapy discovery.
- Discover novel antigens for tumor targeting
- Screen B-cell hybridomas against antigens
- Identify relevant receptors on tumor cells
Learn More about the ZE5 Cell Analyzer »
Learn more about CAR T-cell development with flow cytometry »
A Flow Cytometer Built for Automation and High-Throughput Screening
Achieve automated high-throughput screening by integrating the ZE5 Cell Analyzer into any robotic automation system such as Propel Labs’ Ascent and Biosero’s Green Button Go.
Confidently Assess CAR Expression
With multiple domains that provide different intra- and extracellular functions, the chimeric antigen receptor or CAR part of the CAR T-cell therapy is a complex protein construct that can be challenging to optimize.
To help you efficiently iterate through and evaluate multiple CAR designs, we offer Stain-Free Western Blotting, a reliable and quantitative method for evaluating CAR protein expression levels.
Stain-Free Western Blotting Delivers:
- Measurement of CAR protein expression using total protein normalization, which enables quantitation over a wide dynamic range
- An expedited workflow which reduces the time to results compared to traditional western blotting protocols
Stain-Free Total Protein Normalization for Western Blotting
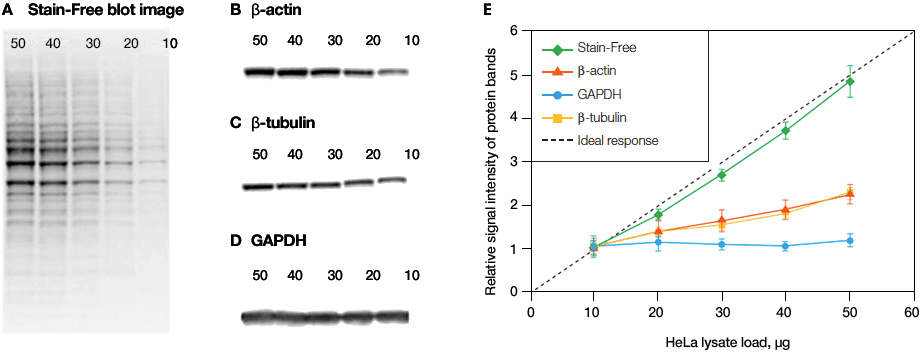
Linearity comparison of Stain-Free total protein measurement and immunodetection of three housekeeping proteins in 10–50 μg of HeLa cell lysate.2 On the left are representative images of (a), Stain-Free blot and the chemiblots for (b), β-actin; (c), β-tubulin and (d), GAPDH. Lane labels correspond to total protein load (μg). Although the actin and tubulin signals appear linear, the densitometric ratio was far below the predicted “quantitative response” of actual loading whereas the Stain-Free signal correlated to the expected result (e).
Resources
Webinars
-
Can We Trust Western Blots?
Learn how to build confidence in western blotting data and tips for producing high-quality western blots in less time.
-
Expert Coffee Chats — Flow Cytometry Optimizations and Applications
Find flow cytometry topics on gene therapy workflows including considerations for consistency across instruments in multiple labs (46:10) and switching from single tubes to plate-based high-throughput analysis (57:20).
Documents
-
A Novel CAR T-Cell Therapy Approach Using Fluorescence-Activated Cell Sorting and Stem Cell Transplantation
Find out how researchers at Columbia University Medical Center developed a new approach for CAR T-cell therapy when faced with the lack of unique antigens.
-
Elucidating Complex Flow Cytometry Studies with the Speed and Sensitivity of the ZE5 Cell Analyzer
Discover how the ZE5 Cell Analyzer obtains biologically relevant data from increasingly complex studies such as exosome analysis and immunophenotyping in a high-throughput manner.
-
Automation of High-Throughput Flow Cytometry with the ZE5 Cell Analyzer
Learn how integrating flow cytometers into automated workcells increases throughput, enables efficient operation, and provides consistent results.
-
An Optimized Workflow for Generating Anti-CD19 CAR T Cells by mRNA Electroporation
See how a CAR vector can be optimized for T-cell expression with the Gene Pulser Xcell System for transfection, followed by analysis with the TC20 Cell Counter, ZOE Cell Imager, and ZE5 Flow Cytometer.
References
- De Plaen E, Lurquin C, Van Pel A, et al. Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum- antigen P91A and identification of the tum- mutation. Proc Natl Acad Sci U S A. 1988;85(7):2274-2278.
- Taylor SC, Posch A. The Design of a Quantitative Western Blot Experiment. BioMed Res Int. 2014;2014:e361590. doi:10.1155/2014/361590
Discover More Cell Therapy Development Solutions
-
Cell Therapy Development Overview
Creating standardized treatments from inherently variable material like living cells requires knowing exactly what you are working with, whether in discovery, development, or manufacturing. Bio-Rad is here to help.
-
Characterization
Develop critical assays to evaluate potency and batch-to-batch consistency, whether for CAR T-cell characterization or development of another cell therapy approach.
-
In Vivo Bioanalysis
Assess the body’s reaction to your cell therapy by developing in vivo bioanalysis assays to monitor CAR T-cell persistence, exhaustion, and other functional parameters.
-
Manufacturing & QC
Efficiently ensure the safety of your cell therapy manufacturing process, whether you are evaluating transgene copy number or verifying the absence of microbial pathogens.







