
On This Page |
Introduction to Biosimilar Development | Biosimilar Workflow | Companion Products for Biosimilar Development | Resources | Citations |
Solutions for Biosimilar Development
We have solutions that provide efficient and cost-effective methods that enable researchers to demonstrate required similarity to the approved reference originator biologic. Explore our solutions for biosimilar development.
Biosimilar Development

The FDA defines a biosimilar as “a product that is highly similar to the reference product notwithstanding minor differences in clinically inactive components; and there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product” (Tierney 2016). More simply, it is a biologic that is almost identical to a previously approved biological product that demonstrates the same clinical safety and efficacy.
To bring a biosimilar to market, developers are required to demonstrate high similarity to the approved biological originator product, including evidence that demonstrates no clinically meaningful differences between the biosimilar and the reference biologic. This is provided as part of the totality of evidence reported to regulatory agencies.
How Similar Does a Biosimilar Need to Be to Its Reference Product?
Although the definition of a biosimilar varies, all regulatory bodies state that a biosimilar must be a biologic, have a biologic reference product — the so-called originator that is licensed based on a non-abbreviated drug submission — and be highly similar to the reference product in terms of safety, quality, and efficacy (Choy and Jacobs 2014). Unlike the production of a chemically identical generic small-molecule drug, biologic manufacturing uses a unique cell line and a complex, proprietary manufacturing process that is impossible to replicate entirely. All biologics have microheterogeneity, e.g., in protein isoforms or glycosylation patterns. Changes to the manufacturing process mean that even an originator biologic may not be 100% identical across production batches. Thus, to ensure that quality, safety, and efficacy are unaffected, biologic products undergo physicochemical and functional comparability experiments before and after manufacturing modifications. Similarly, biosimilar development requires extensive physicochemical and biological characterization of the reference originator product using multiple analytical techniques to ensure similarity. Based on the results of this characterization, preclinical studies and clinical trials are undertaken to resolve uncertainty about the similarity of the new biosimilar product.
Demonstrating Biosimilarity
Although regulatory processes for establishing biosimilarity vary among countries, all require the use of a single reference originator product for comparisons of efficacy, safety, and quality. The biosimilar is submitted for both preclinical studies and clinical trials analogous to those for a new drug approval.
Preclinical Studies
In preclinical studies, comparison is required for the proposed vs. the reference originator product to examine protein structure, enzymatic structure, posttranslational modifications, potential protein variations, and intentional chemical modifications in multiple representative lots. In vitro, in vivo, and functional assays should demonstrate that the proposed product has highly similar biologic activity and potency and no clinically meaningful differences from the reference product (Ventola 2013).
Clincal Trials
Authorities generally agree that data on human pharmacokinetic/pharmacodynamic (PK/PD) responses are fundamental for supporting biosimilarity and require a clinically relevant study population, dose, and route of administration.
The design of comparative clinical studies for biosimilars to investigate efficacy, safety, tolerability, and immunogenicity vary based on the findings and limitations of previous testing, the extent to which human PK/PD data predict clinical outcomes, and the extent of clinical experience with the reference originator and proposed biosimilar. An equivalence design is usually used to confirm that the proposed biosimilar is neither inferior nor superior to its reference originator product. Assessment of immunogenicity is critical because it can alter the PK or promote development of antidrug antibodies, thereby affecting safety and efficacy of the proposed drug. Because the required sample size for clinical trials is smaller for biosimilars than for originator biologics, careful monitoring of immunogenicity and other side effects is important in post-marketing surveillance of biosimilars (US FDA 2015).
Biosimilar Development Workflow

Purification
The biggest challenge facing the biosimilar developer is to produce a final product at a lower cost than the originator. To find the best purification strategy and migrate the workflow from lab to process scale requires reliable instruments, columns, and resins. It’s important to control costs and minimize steps while maintaining the purity, efficacy, and structure of the biosimilar.
Solutions for Purification »
Quantification of Impurities
Biosimilars must demonstrate comparability in structure, function, and efficacy to their originator biologic. There is a need to understand the underlying mechanism of action of the originator as well as the biosimilar in order to design a cell-based assay that measures comparability. The resulting data needs to be precise, accurate, and reproducible.
Solutions for Quantification of Impurities »
Comparability
Biosimilars must demonstrate comparability in structure, function, and efficacy to their originator biologic. There is a need to understand the underlying mechanism of action of the originator as well as the biosimilar in order to design a cell-based assay that measures comparability. The resulting data needs to be precise, accurate, and reproducible.
Solutions for Comparability »
Characterization
To ensure that there are no clinically meaningful differences, a biosimilar is compared to its reference product for safety, efficacy, and immunogenicity, including the titer and reactivity of anti-drug antibodies (ADAs). Reagents used for this portion of development need to meet high standards so that the resulting data is robust.
Solutions for Characterization »
Companion Products for Biosimilar Discovery

NGC Chromatography Systems
- Quickly optimize purification methods using configurable scouting and automated multi-D features
- Easy method creation to screen multiple resin types in order to identify optimal resin chemistry for the removal of product impurities

Chromatography Resins
- Choose from a range of scalable, economical resins designed to suit your purification strategy
- Increase yield and decrease process time by utilizing higher binding capacity

Gel Imagers
- Flexibility to address multiple imaging needs such as multiplex fluorescence or chemiluminescence detection in addition to DNA or protein gel imaging
- Assess purity using the gold standard densitometer with automated calibration
- Security edition software module for 21 CFR 11 compliance and an Installation and Operations Qualification (IQ/OQ) Kit, allowing for easy transition from discovery to a regulated environment
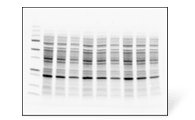
Stain-Free Gels
- Visualize protein purity in under 30 minutes without a staining protocol
- Multiple protein gel formulations and sizes for SDS-PAGE and native PAGE applications

Droplet Digital PCR (ddPCR) Systems, Primers, Assays, and Arrays
- Characterize the cellular universe using multiparameter cell analysis to identify and validate biomarkers
- Monitor up to 30 parameters simultaneously, using up to 5 lasers, and identify cell subtypes faster and with more precision
- Fast data acquisition rate allows rare or transient cell types to be analyzed
- Small-particle detection to analyze biomarkers in exosomes
-
Host Cell Protein Workflow
- Sort and analyze cells at your bench to support biomarker discovery and validation assays
- Up to 3 lasers provide the flexibility needed to distinguish & sort subtly different cell populations
- Cells are sorted quickly and gently while maintaining sensitivity and precision for downstream assays

Antibodies
- A full range of off-the-shelf antibodies including PrecisionAb Validated Western Blotting Antibodies

S3e Cell Sorter
- Sort cells expressing fluorescent markers
- 2-way cell sorting

ZE5 Cell Analyzer
- Analysis of up to 30 parameters from a single sample expanding the number of monitored biomarkers by a cell based assay
- Measures rare or transient cell populations quickly

HuCAL Custom Monoclonal Antibodies
- Customizable, fully-human, animal-free antibodies available
- Highly specific, high affinity, recombinant antibodies with a greater than 90% success rate in only 8 weeks
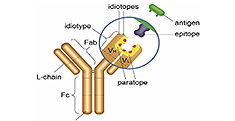
Anti-Idiotypic Antibodies
- Ready-made recombinant, anti-biosimilar antibodies available in multiple formats
- Fully human, validated, and perfect as controls or calibrators for ADA assays
Resources
Documents

What's New In US Biosimilar-Land?
(PDF 377 KB)
Biosimilars: Are They Ready for Primetime?
(PDF 270 KB)
Biosimilars: Providing More Treatment Options
(PDF 152 KB)

Reliable, Streamlined 2-D Western Blot Workflow for Evaluation of Antibodies Developed for Detection of Host Cell Proteins
(PDF 1.82 MB)
Reliable Single-Cell Sorting with Bio-Rad's S3e Cell Sorter
(PDF 242 KB)
Development of a Non–Affinity Based Purification Platform for Neutral/Basic IgMs
(PDF 242 KB)
Monoclonal Antibody Purification Using UNOsphere SUPrA Media
(PDF 242 KB)

A Biosimilars Workflow: From Purification to Characterization
(PDF 1.79 MB)
NGC Fraction Collector Brochure
(PDF 879 KB)
ChemiDoc Imaging Systems
(PDF 295 KB)
Selecting the Optimal Resins for Monoclonal Antibody Process Purification
(PDF 290 KB)
Simplified Long Noncoding RNA Discovery Brochure
(PDF 697 KB)
Fluorescent Western Blotting Antibodies
(PDF 247 KB)
Citations
Choy E and Jacobs IA (2014). Biosimilar safety considerations in clinical practice. Semin Oncol 41 Suppl 1, S3–14. PMID: 24560025
DiMasi JA et al. (2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ 47, 20–33. PMID: 26928437
United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research (2015). Scientific considerations in demonstrating biosimilarity to a reference product: Guidance for industry.
Tierney L (2016). Live from PDA/FDA: an overview of FDA’s expectations of biosimilars. Healthcare Packaging, Sept. 14.
Ventola CL (2013). Biosimilars: part 2: potential concerns and challenges for p&t committees. P T 38, 329–335. PMID: 23946628
Visiongain (2016). Biologics Market Trends and Forecasts 2016–2026.
