Solutions for Successful Biosimilar Characterization
We have solutions that provide efficient and cost effective methods to assist in the characterization of biosimilar drug products.


Biosimilar Characterization Methods
As part of the drug development program, the biosimilar is compared to the reference product to ensure that there are no clinically meaningful differences in terms of safety, efficacy, and immunogenicity, including the titer and reactivity of anti-drug antibodies (ADA) produced in vivo. The results form part of the totality of evidence required by regulatory agencies. These data must be robust; therefore the reagents used must meet high standards.
Bio-Rad’s antibiotherapeutic antibody reagents for ADA and PK assay development are selected in vitro from the synthetic Human Combinatorial Antibody Libraries (HuCAL). Guided selection strategies enable the generation of fully human, highly specific inhibitory and noninhibitory anti-idiotypic antibodies and specialized drug-target complex binders. These different types of antibodies enable development of PK assays to detect free or total drug, or drug bound to its target. Additionally, the inhibitory antibodies can be used as reference standards in an ADA assay, replacing the need to source antibodies raised in animals.
Bio-Rad provides an expanding portfolio of antibodies against marketed monoclonal antibody drugs and a custom service for additional specificities. Our QC process incorporates several relevant published best practices, aiding with critical reagent life cycle management and characterization and the maintenance of optimal assay performance over time.
Companion Products for Biosimilar Characterization

HuCAL Custom Monoclonal Antibodies
- Customizable, fully-human, animal-free antibodies available
- Highly specific, high affinity, recombinant antibodies with a greater than 90% success rate in only 8 weeks
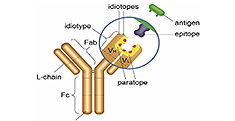
Anti-Idiotypic Antibodies
- Ready-made recombinant, anti-biosimilar antibodies available in multiple formats
- Fully human, validated, and perfect as controls or calibrators for ADA assays
