Bio-Rad provides a comprehensive range of products for COVID-19 diagnosis and confirmation including real-time PCR, droplet digital PCR, rapid antigen testing, and serological testing. Our SARS-CoV-2 testing solutions span the full course of the viral infection, with options from active infection testing to post-infection antibody testing.
Bio-Rad provides a comprehensive range of products for COVID-19 diagnosis and confirmation including real-time PCR, droplet digital PCR and rapid antigen testing. Our SARS-CoV-2 testing solutions span the full course of the viral infection, with options from active infection testing to post-infection antibody testing.
Our COVID-19 diagnostic kits support detection of the Omicron variant. Learn More »
Some products have limited regional availability. Please contact your local sales office contact your local sales office for any product availability questions.
SARS-CoV-2 Testing Over the Course of Viral Infection

-
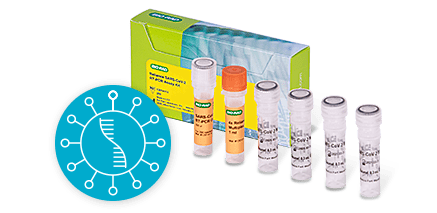
COVID-19 RT-PCR Molecular TestReliance SC2/Flu A/Flu B RT-PCR Kit (IVD)
We offer two COVID-19 RT-PCR molecular in vitro diagnostic tests to detect SARS-CoV-2:
- Reliance SARS-CoV-2/Flu A/Flu B RT-PCR Assay Kit (IVD) — detects and differentiates Flu A and Flu B from COVID-19 by targeting nucleocapsid Flu A M1 and Flu B NS2 genes.
- Reliance SARS-CoV-2 RT-PCR Assay Kit (IVD) — detects COVID-19 by targeting nucleocapsid N1 and N2 genes.
-
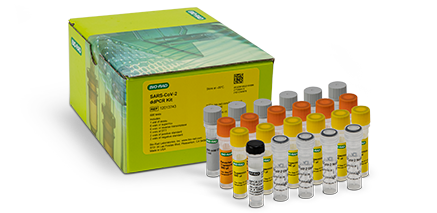
COVID-19 ddPCR Molecular TestSARS-CoV-2 Droplet Digital PCR (ddPCR) Kit
Our SARS-CoV-2 ddPCR Kit is a partition-based endpoint RT-PCR test for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharyngeal anterior nasal and mid-turbinate nasal swab specimens and nasopharyngeal wash/aspirate plus nasal aspirate specimens from patients suspected of being infected with COVID-19 by a healthcare provider.
-
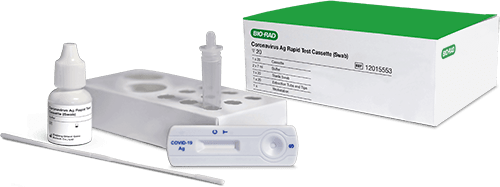
COVID-19 Rapid Antigen TestCoronavirus Ag Rapid Test Cassette (Swab)
Our Coronavirus Ag Rapid Test is a quick and easy tool for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasal or nasopharyngeal swab specimens for the diagnosis of COVID-19 infection.
For COVID-19 diagnosis and confirmation, Bio-Rad offers a 15-minute SARS-CoV-2 antigen test for rapidly diagnosing patients in laboratory and point-of-care settings. A complementary Control Swabs Kit can be used to validate new test kits, lots, and operators.

COVID-19 RT-PCR Molecular TestReliance SARS-CoV-2 RT-PCR Assay Kit*
We offer one COVID-19 RT-PCR molecular in vitro diagnostic test in the U.S. to detect SARS-CoV-2 under EUA.
Reliance SARS-CoV-2 RT-PCR Assay Kit — detects COVID-19 by targeting nucleocapsid N1 and N2 genes.

COVID-19 ddPCR Molecular Test SARS-CoV-2 Droplet Digital PCR (ddPCR) Kit*
Our SARS-CoV-2 ddPCR Kit is a partition-based endpoint RT-PCR test for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharyngeal anterior nasal and mid-turbinate nasal swab specimens and nasopharyngeal wash/aspirate plus nasal aspirate specimens from patients suspected of being infected with COVID-19 by a healthcare provider.
COVID-19 Detection Kits are unlikely to be impacted by mutations in Omicron variant.
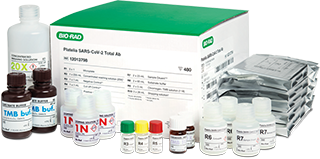
COVID-19 Serology Test Platelia SARS-CoV-2 Total Ab Assay
Our qualitative serology test detects total anti-nucleocapsid antibodies (IgM, IgA, and IgG) to SARS-CoV-2 in human serum and plasma to aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. This blood-based immunoassay, designed for in vitro diagnostic testing, was granted U.S. Emergency Use Authorization in 2020 to test for total antibodies in a single assay.*
COVID-19 Serology Testing
Platelia SARS-CoV-2 Total Ab Assay
Our COVID-19 serology assay aides in diagnosing SARS-CoV-2 infections when RT-PCR was not used as a first-line diagnostic tool, as when samples are collected more than 8 days after symptom onset. The test can also be used for suspected COVID-19 infections, particularly in patients hospitalized with severe symptoms who have tested negative with RT-PCR.
The Platelia SARS-CoV-2 Total Ab assay detects total anti-nucleocapsid antibodies (IgM, IgA, and IgG) to SARS-CoV-2 with high sensitivity (92% sensitivity for patients tested ≤ 8 days after symptom onset and 100% for patients tested >8 days after symptom onset).
This blood-based immunoassay, designed for in vitro diagnostic testing, is the first serology assay with U.S. Emergency Use Authorization to test for total antibodies in a single assay. The test has also met requirements for applying the CE-Mark in Europe.
Legal Notice:
Europe: The Reliance SARS-CoV-2 RT-PCR Kit (IVD) and the Reliance SC2/Flu A/Flu B RT‑PCR Kit (IVD) is for use in the European Union only with CE-IVD marked In Vitro Diagnostic Medical Devices. These kits are CE Marked for sale outside the U.S., with limited regional availability. Please check with your local sales office.
The CFX96 Dx System is intended for use in the fields of research and human in vitro diagnostics. The system is CE-IVD marked in compliance with the European Union diagnostic medical device manufacturing standards.
Europe: For in vitro diagnostic use. The CFX96 Dx System and CFX96 Deep Well Dx System meet the requirements of the In Vitro Diagnostic Medical Devices Directive (98/79/EC). The CE IVD, CFX96 Dx and CFX96 Deep Well Dx Systems are for distribution and use in specific European countries only (Austria, Belgium, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Malta, Netherlands, Poland, Portugal, Slovenia, Spain, and Sweden).
USA: For In Vitro Diagnostic Use. The CFX96 Dx and CFX96 Deep Well Dx Systems are registered with the US FDA as Class II 510(k) exempt devices.
China: The CFX96 Deep Well Dx System is registered for In Vitro Diagnostic use.
Worldwide: The CFX96 Dx and CFX96 Deep Well Dx Systems are registered for In Vitro Diagnostic use in the following countries: Argentina, Australia, Belarus, Bolivia, Brazil, Brunei, Chile, Colombia, Costa Rica, Dominican Republic, Egypt, Hong Kong, India, Laos, Morocco, New Zealand, Norway, Pakistan, Philippines, Qatar, S. Korea, Saudi Arabia, Singapore, Switzerland, Taiwan, Thailand, The Netherlands, Turkey, United Kingdom, Ukraine, Vietnam, and Yemen.
These products and/or their use are covered by claims of U.S. patents, and/or pending U.S. and non-U.S. patent applications owned by or under license to Bio-Rad Laboratories, Inc. Purchase of a product includes a limited, non-transferable right under such intellectual property for use of the product for internal research and diagnostic purposes only. No rights are granted for use of a product for commercial applications of any kind, including but not limited to manufacturing, quality control, or commercial services, such as contract services or fee for services. Information concerning a license for such uses can be obtained from Bio-Rad Laboratories. It is the responsibility of the purchaser/end user to acquire any additional intellectual property rights that may be required.
Kits and reagents are sold for research use only. Not for use in diagnostic procedures.
BIO-RAD is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions.
