
- Share on:
- Bio-rad Twitter
- Bio-rad Facebook
- Bio-rad LinkedIn
The Power of Biotherapeutics: Innovative Approaches and Future Directions
Over the past few years, significant progress has been made in the development of innovative biotherapeutic products aimed at addressing unmet medical needs. The pharmaceutical industry's focus resulted in the development of advanced therapeutic modalities, including monoclonal antibodies, cell and gene therapies, and oligonucleotide therapeutics. Such products have in turn provided new avenues for treating debilitating conditions, such as cancer, autoimmune disorders, infectious diseases, and genetic diseases. Biotherapeutics are unique in their method of action, which involves targeting specific proteins, genes, or cells in the body to achieve therapeutic effects. As a result, they have emerged as a rapidly growing area of research and development contributing to the evolution of the pharmaceutical industry. This article explores the primary focus areas for the industry, highlighting different mechanisms of action and the potential of novel biotherapeutics to change the future of medicine.
Biotherapeutics, also known as biologics or biological drugs, have been around for decades, with insulin serving as a prime example. Empowered by technological advancements, we continue to delve deeper into complex biological processes and learn more about catalysts of disease. This knowledge has revolutionized the way we approach medicine, paving the way for novel biotherapeutics designed to bind to specific targets within cells with extreme precision (Jallal 2017). Unlike synthetic small molecule drugs, biotherapeutics are most often composed of large, complex molecules that include combinations of cellular components like proteins, peptides, nucleic acids, and carbohydrates. They are also typically derived from living cells or microorganisms that can be designed to produce molecules of interest.
Considering the nearly endless capabilities of biotherapeutics, it is no surprise that biotherapeutics now form approximately 40 percent of all new medicines approved by the U.S. Food and Drug Administration (FDA) (Lim 2023). Cancer research is currently on top of the league when it comes to the industry's therapeutic areas of interest, with over 7,000 drugs in its pipeline (Pharma R&D Annual Review 2022). This is unlikely to change, considering oncology is projected to be the therapeutic area with the highest spending by 2027, with a forecast of $377 billion. (Statista 2023).
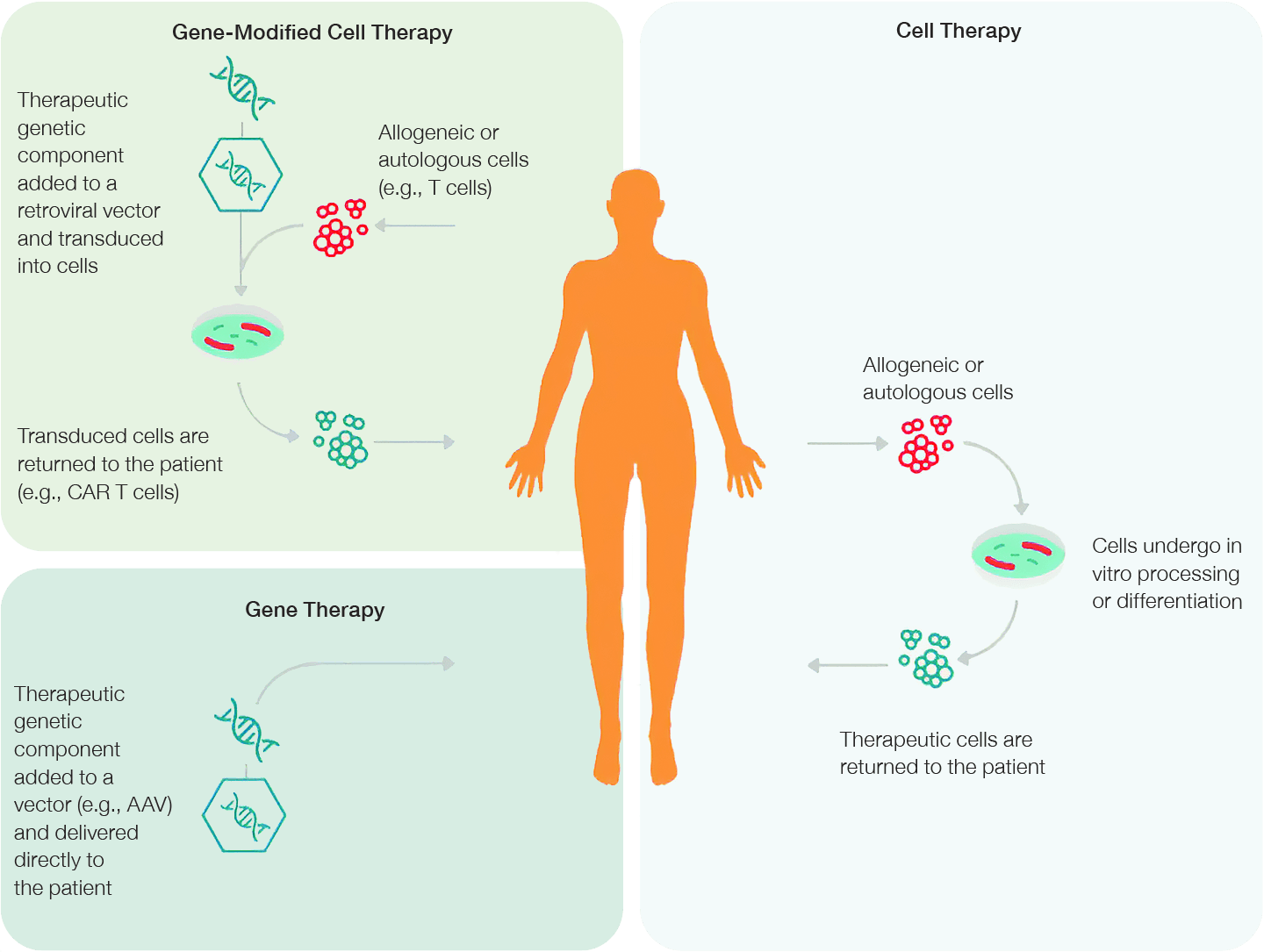
Fig. 1. CGT approaches. Gene therapies aim to treat conditions at a genetic level through the methods of gene augmentation, gene suppression, or gene editing.
An essential step in developing a successful CGT product relies on the safe and effective delivery of genetic material or modified cells into a patient's body to treat, prevent or potentially cure a disease (Pratt 2022). Cell or gene-modified cell therapies use cells derived from either the patient (autologous) or a healthy donor (allogeneic). These cells are then isolated, cultivated, or, in the case of gene-modified cell therapies (CAR T-cell therapy, for example), manipulated ex vivo and transplanted back into the patient. Gene therapies, on the other hand, use viral or non-viral vectors to deliver therapeutic genetic material directly into a specific cell. This approach aims to correct the overall function of the target cell or protein at a fundamental DNA level by gene augmentation, gene suppression, or gene editing (Figure 2).
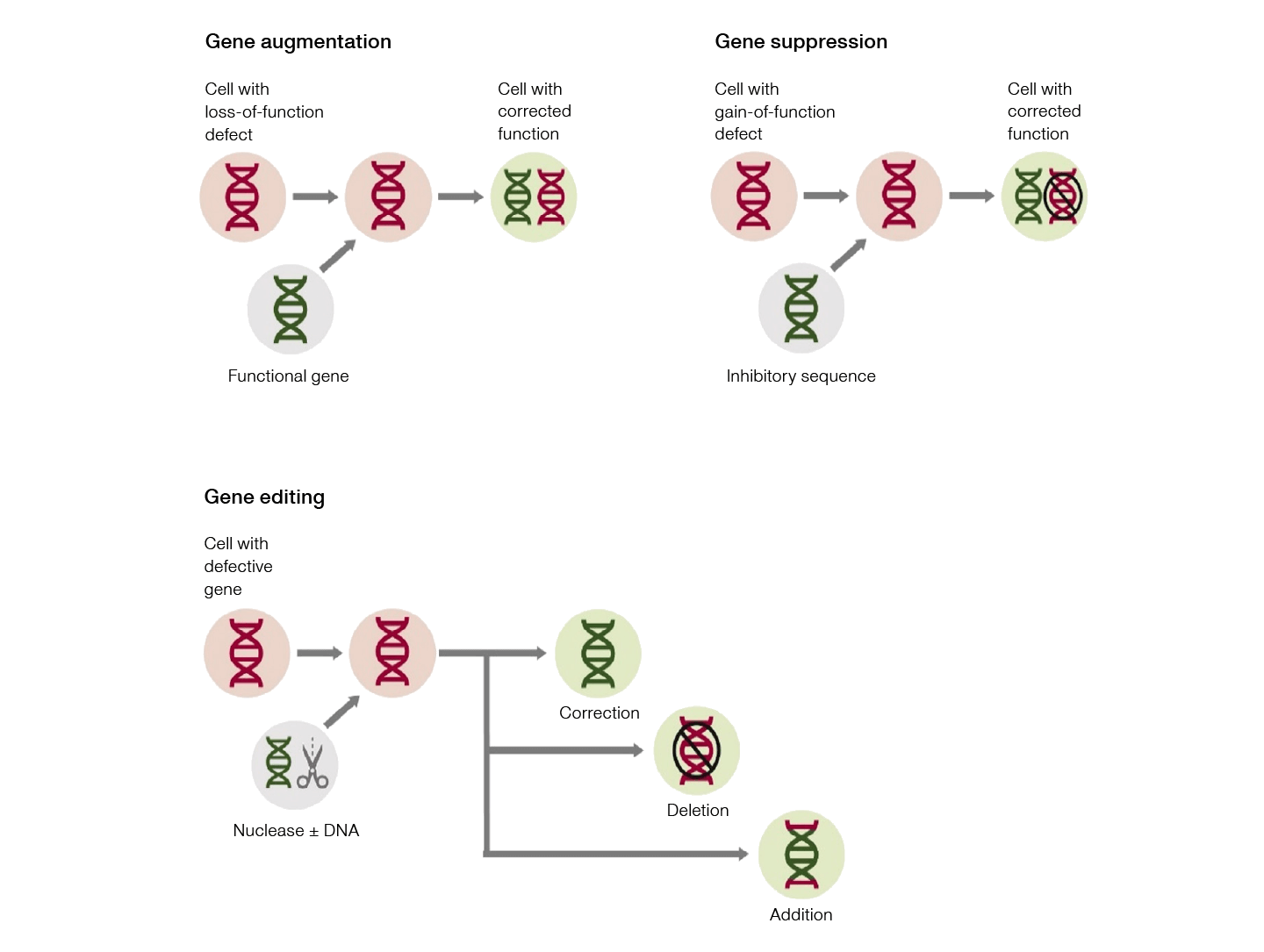
Fig. 2. Gene therapy approaches. Advances in gene-modified cell therapy, gene therapy, and cell therapy have vastly expanded the capabilities and applications of CGT products.
Gene augmentation occurs when a functional copy of a defective gene is delivered to a target cell, enabling the cell to resume its correct function. On the other hand, genetic conditions, such as Huntington's disease, caused by overexpression of certain genes or noncoding RNA, can be treated by gene suppression. This can occur both transcriptionally, by “silencing” a specific DNA sequence, or post-transcriptionally, by targeting a specific mRNA sequence. Editing defective genes in situ to restore “normal” function, using genome editing tools such as CRISPR-Cas9 and zinc finger nucleases (ZFNs) is another promising gene therapy strategy ushering in a new era for CGTs.
Recombinant adeno-associated adenovirus (AAV) is often the vector of choice when it comes to in vivo gene delivery — mainly due to it being less immunogenic relative to other viruses, its ability to infect a wide range of cells, and overall safety. The first AAV–mediated gene therapy product, Luxturna (Spark Therapeutics, Inc.), was approved by the FDA in 2017. Luxturna provides a functional copy of the retinal pigment epithelium–specific 65 (RPE65) gene to retinal cells, effectively restoring normal function and preventing blindness in individuals with biallelic RPE65 mutation–associated retinal dystrophy (Rodrigues et al. 2018).
More recently, Zolgensma (Novartis Pharmaceuticals) received FDA approval in 2019 for the treatment of spinal muscular atrophy (SMA), a disease that results in muscle weakness and atrophy. SMA is the leading inherited cause of infant death (Waldrop et al. 2020), with the most common type arising from the biallelic loss-of-function of the survival motor neuron 1 gene (SMN1). Zolgensma works by administering an SMN transgene, from which a fully functional SMN protein can be produced (Waldrop et al. 2020).
Both Luxturna and Zolgensma demonstrate the power of gene augmentation therapy. By delivering a functional copy of the gene (cDNA) to a specific cell type, such therapies restore protein function and improve patient outcomes.
The advent of RNA technology has unlocked new possibilities for a variety of therapeutic modalities, such as vaccines, cancer immunotherapy, and gene editing. As such, it provides an attractive platform for gene therapy. For example, mRNA–mediated therapeutics offer several advantages over DNA–mediated therapy. For one, mRNA does not integrate into the genome, further minimizing the risk of insertional mutagenesis from delivering exogenous DNA fragments. Additionally, as mRNA does not need to enter the nucleus to function, protein translation of a transgene begins as soon as the mRNA reaches the cytoplasm.
The recent success of COVID-19 mRNA vaccines delivered in lipid nanoparticles (LNPs), for example, has undoubtedly reignited interest in the development of mRNA vaccines for other infectious diseases and malignancies. Nonviral vectors such as LNPs and polymers have gained popularity as delivery vessels for drugs and nucleic acids. Specifically, LNPs have successfully been used in gene therapy delivery approaches due to their low immunogenicity, very low cytotoxicity (if any), large packaging capabilities, and cost- and time-effective manufacturing processes (Paunovska et al. 2022).
In the realm of cutting-edge therapeutics, RNA–mediated treatments are on the rise. Exciting developments include classes of small RNA molecules, such as small activating RNA (saRNA) and circular RNA (circRNA). saRNA is engineered to upregulate specific gene expression and, therefore the production of the corresponding protein. Early studies have shown promising results, indicating that saRNA could be a game-changing approach to treating a variety of diseases (Kwok et al. 2019). Unlike linear RNA molecules, circRNA forms a covalently closed loop structure, rendering them more pharmaceutically stable than linear RNA and insusceptible to degradation by exonucleases. This unique feature and its involvement in many biological processes, make it an attractive candidate for various biomedical applications (Liu et al. 2022).
Additional RNA–based technologies in development include the use of mRNA interference (RNAi). This naturally occurring mechanism can result in gene silencing, either by preventing protein translation or by causing mRNA degradation. Common RNAi molecules are microRNA (miRNA), short interfering RNA (siRNA), and short hairpin RNA (shRNA) (Forterre et al. 2020). Of these, shRNAs are the only molecules capable of genome integration and could therefore be used for stable, long-term silencing in gain-of-function genetic diseases. shRNAs are composed of two short complementary RNA molecules connected by an even shorter linker sequence, forming a hairpin. Several preclinical and clinical studies are currently underway to investigate the potential of shRNA molecules for solid tumor treatments, with an initial focus on liver cancer and gliomas (Akin et al. 2019).
While the potential of such RNA–mediated therapies appears nearly limitless, further research is needed to improve understanding of their mechanisms of action, safety implications, and potential applications. With ongoing study and development, RNA–based treatments could transform the CGT landscape.
Antisense oligonucleotides (ASOs), made up of short single-stranded DNA or RNA, have emerged as a promising tool for gene silencing. ASOs display exceptional specificity to target mRNA sequences and can be used for a plethora of difficult-to-treat diseases. Recently, several ASO-mediated gene therapy products have been approved by the FDA (such as eteplirsen from Sarepta Therapeutics, Inc. for the treatment of Duchenne muscular dystrophy), and several clinical trials are underway (for example, those studying inherited retinal disorders) (Del Pozo-Rodriguez et al. 2020). The growing number of ASO–based clinical trials for various diseases and malignancies underscores the potential of this technology to transform medicine.
Therapeutic antibodies are another rapidly expanding class of drugs thanks to their excellent specificity, high affinity, long in vivo half-life, and potent agonistic or antagonistic properties (Harth et al. 2018). These features have made them valuable tools for targeting specific proteins or cells within the body with the potential to treat a wide range of diseases, including cancer, autoimmune disorders, and infectious diseases. New innovations in discovery and engineering methods are leading to more precise and effective antibody-based therapies, paving the way for new treatments that hold great promise for improving patient outcomes.
Monoclonal antibodies (mAbs) are one of the most promising classes of biotherapeutics due to their high specificity, affinity, and potent therapeutic properties. Supportive government initiatives coupled with an increasing demand for personalized medicines have positioned mAbs as a major focus of R&D spending. The global mAbs market is projected to reach over $495 billion at a compound annual growth rate (CAGR) of 11 percent by 2030 (Grand View Research 2022).
A key advantage of mAbs is their ability to target specific proteins on the surface of cancer cells, either by blocking cell growth signals or by flagging the cancer cell for destruction by the immune system. An example is rituximab (Genentech), a chimeric mAb that selectively binds to B-cell protein CD20, triggering cell death. Rituximab is used to effectively manage and improve the outcome of B-cell malignancies, including chronic lymphocytic leukemia and non-Hodgkin lymphoma. However, as rituximab is a chimeric mouse/human antibody, the mouse portion can cause unwanted immunogenicity in humans. While humanized antibodies have been able to reduce the issue of immunogenicity, fully human antibodies are far less immunogenic and more clinically effective, rendering them more desirable for future biotherapeutics (Bryon-Dodd 2022).
Antibody therapies, often involving mAbs, are also being developed to treat autoimmune disorders such as rheumatoid arthritis (RA), lupus, and psoriasis. These therapies work by targeting molecules involved in inflammation and immune system activation (Frank 2016), and the main types include:
- Tumor necrosis factor (TNF) inhibitors: TNF is a critical pro-inflammatory cytokine typically produced in response to acute and systemic inflammatory events. In RA and other autoimmune conditions, TNF may be produced in excess, triggering chronic inflammation. TNF inhibitors bind to TNF, rendering it inactive and driving immune suppression
- B-cell inhibitors: B cells regulate immune responses through antibody production and T-cell activation. B-cell inhibitors specifically target a protein that initiates B-cell activity, thus reducing the overall activity of the immune system, which can ease symptoms of autoimmune diseases
- T-cell inhibitors: Similar to B cells, T cells also play an essential role in immune system response and inflammation. T-cell inhibitors work by blocking a protein responsible for stimulating the excess production of T cells
- Interleukin (IL) inhibitors: ILs are a large group of cytokines involved in the activation, differentiation, and function of different types of immune cells. ILs have both pro- and anti- inflammatory properties, making them crucial for immune system regulation and inflammation. Other IL inhibitors function by blocking the action of specific ILs, inhibiting the activation of immune cell types and reducing inflammation
Blocking or inhibiting inflammation can ease symptoms associated with autoimmune conditions. However, immune system suppression is accompanied by various side effects and significantly increases a patient's risk of infection. Therefore, careful patient monitoring during the administration of these therapies is required to avoid serious complications.
Combination therapies harness two or more different biotherapeutics to improve the efficacy of the treatment and minimize the risk of drug resistance. In recent years, various biotherapeutic agents, such as antibody-drug conjugates (ADCs), CAR T cells, and oligonucleotides, have been explored as potential components of combination therapies.
With the potential to revolutionize cancer treatment, ADCs combine the specificity of mAbs with the power of a potent cytotoxic payload. Unlike conventional chemotherapy, the mAb component of ADCs is designed to specifically target and bind to a tumor-associated antigen (TAA), releasing the cytotoxic payload at the tumor site (Figure 3). This highly targeted approach aims to minimize systemic exposure to cytotoxic drugs and damage to healthy cells, widening their therapeutic index. ADCs can also be designed to target different TAAs or to deliver different cytotoxic payloads, allowing for a tailored treatment approach that addresses the heterogeneity of tumor cells within a patient (Fu et al. 2022).

Fig. 3. Antibody-drug conjugate structure and characteristics. Distinct features of an antibody-drug conjugate target a cytotoxic drug payload to diseased cells with high spatial and temporal precision. Image adapted from Fu et al. 2022 under a CC BY 4.0 license.
Similarly, bispecific antibodies (bsAbs) are another important addition to the broadening reach of immunotherapy. bsAbs are designed to simultaneously bind to two different proteins to bring them in close proximity, which, depending on the mechanism of action, can lead to a variety of effects. A bsAb which helped spur clinical interest in harnessing this approach linking an effector to a target cell is blinatumomab (BLINCYTO, Amgen Inc.), a bispecific T-cell–engager (BiTE) which first received FDA approval in 2014 and European Medicines Agency (EMA) approval in 2015. BiTE molecules work by inducing proximity at the cellular level, bringing T cells together with tumor cells so that the former can recognize and destroy the latter (Figure 4). Blinatumomab, for example, is an anti-CD19 x anti-CD3 BiTE molecule and is currently being investigated for the treatment of acute lymphoblastic leukemia (Moriarty 2022).
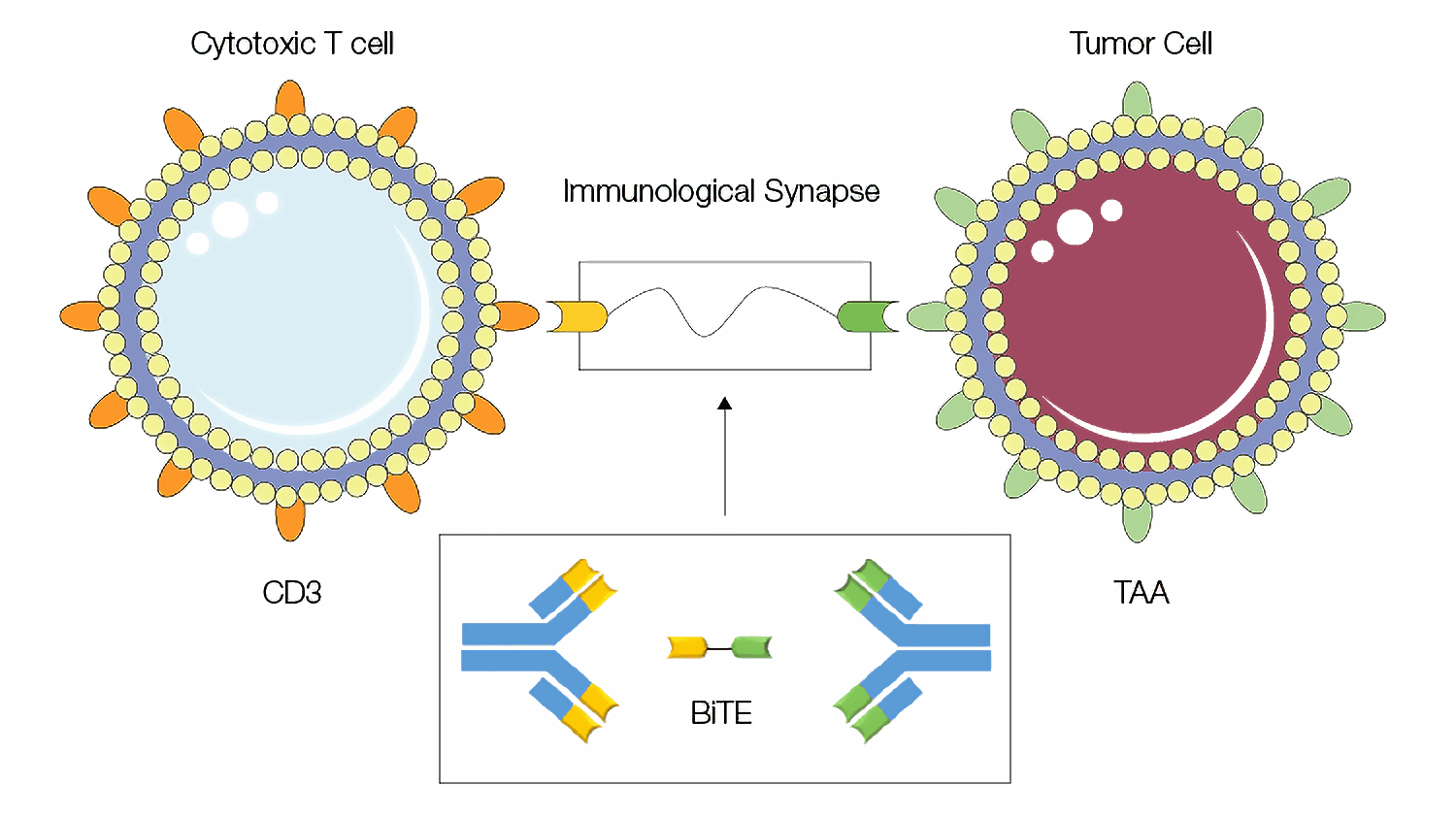
Fig. 4. Bispecific T-cell–engager (BiTE) molecules mode of action. The BiTE approach uses bispecific antibodies to form an immunological synapse, bringing two cells in close proximity. Image adapted from Kamta et al. 2017 under a CC BY 4.0 license.
Another widely popular and fast-growing immunotherapy platform employs CAR T cells, which have shown significant promise in the treatment of hematologic malignancies. These therapies involve genetically modifying a patient's T cells to express chimeric antigen receptors (CARs) that specifically target TAAs. While these therapies have demonstrated impressive clinical outcomes, many patients ultimately relapse due to antigen loss or resistance to the treatment (Xu et al. 2019). One approach to addressing this challenge is to combine CAR T-cell therapies with other treatments, such as immune checkpoint blockade therapy, which could potentially achieve functional persistence of CAR T cells (Grosser et al. 2019).
To enhance the efficacy and specificity of drug delivery, combination therapies harnessing modified LNPs to deliver combinations of drugs to target cells have gained significant attention in recent years. For example, one of the biggest challenges in RNA–based therapeutics is developing effective strategies for in vivo delivery of miRNAs or siRNAs to solid tumors. Combining a nanoparticle with a tumor-targeting ligand offers a promising solution. For example, a modified LNP possessing a tumor-targeting, single-chain antibody fragment (scFv), was shown to specifically target metastatic lung tumor cells delivering both siRNA and miRNA. This, in turn, elicited an enhanced anticancer effect — ultimately leading to tumor growth inhibition (Chen et al. 2010). An equally positive therapeutic response was observed to the combinatorial delivery of siRNA and miRNA to lung adenocarcinoma cells via a lung-targeting, polymer-based nanoparticle (Xue et al. 2014).
A similar approach was employed to direct RNA–based therapeutics to immune cells, generating numerous novel opportunities for therapeutic interventions. Specifically, conjugating a CD4 antibody to LNPs was shown to enable specific targeting and mRNA interventions to CD4+ cells, including T cells, which are usually resistant to transfection by exogenous mRNA (Tombácz et al. 2021). This CD4–targeted mRNA–LNP platform could improve treatment outcomes for a wide range of diseases by facilitating in vivo manipulation of all T cell subtypes in difficult-to-access tissues, such as lymph nodes. Additionally, targeted delivery of engineered genome-editing enzymes via this platform has the potential to cure human immunodeficiency virus (HIV) by removing the integrated provirus from the genome of latently infected cells (Tombácz et al. 2021). This approach represents a significant step forward in the development of novel RNA–mediated immunotherapies.
Although combination therapies undoubtedly have huge potential, challenges associated with dosing, effectivity and safety must be investigated further before such treatments can be widely adopted.
Biotherapeutics have emerged as a revolutionary class of therapeutic products that offer significant benefits over traditional small-molecule drugs. They have already shown great promise in treating a variety of diseases, including cancer, genetic diseases, and autoimmune disorders, and there is enormous potential for continued growth and advancement in this field. The industry is in the midst of a dynamic and innovative phase of growth, with the discovery of exciting new targets and methods of action driving further advances in immunotherapies, ADCs, bsAbs, and CGTs. Moreover, the rise of combination therapies that incorporate different biotherapeutic agents is a fascinating area of research that could transform how we target disease, setting the stage for a new era of precision medicine.
Akinc et al (2019). The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 14, 1084-1087.
Approved cellular and gene therapy products. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products, accessed March 09, 2023.
Bryon-Dodd (2022). A pioneering approach to biotherapeutic antibody discovery. bioradiations.com/pioneering-biotherapeutic-antibody-discovery-1022/, accessed March 08, 2023.
Chen et al (2010). Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 18(9), 1650-1656. DOI: 10.1038/mt.2010.136.
Del Pozo-Rodríguez A et al. (2020) Gene Therapy. Adv Biochem Eng Biotechnol. 171, 321-368.
Forterre et al (2020). A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers. 12, 1852. DOI: 10.3390/cancers12071852.
Frank (2016) Biologics for RA and other autoimmune conditions. arthritis-health.com/treatment/medications/biologics-ra-and-other-autoimmune-conditions, accessed March 08, 2023.
Fu et al (2022). Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Nature., 7(:93), DOI: 10.1038/s41392-022-00947-7.
Gottleib S (2018). Capturing the benefits of competition for patients. fda.gov/NewsEvents/Speeches/ucm599833.htm, accessed March 08, 2023.
Grand View Research (2022). Monoclonal antibodies market worth $494.53 billion By 2030. grandviewresearch.com/press-release/global-monoclonal-antibodies-market, accessed March 08, 2023.
Grosser et al (2019). Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer cell, 36(5), 471–482. DOI: 10.1016/j.ccell.2019.09.006.
Harth et al (2018). Generation by phage display and characterization of drug-target complex-specific antibodies for pharmacokinetic analysis of biotherapeutics. mAbs, 11(:1), 178-190, DOI: 10.1080/19420862.2018.1538723.
Jallal (2017). Realizing the promise of biologics. hhpronline.org/volume-16-issue-1-2/2017/4/9/realizing-the-promise-of-biologics, accessed March 08, 2023.
Kwok et al (2019). Developing small activating RNA as a therapeutic: current challenges and promises. Ther Deliv. Therapeutic delivery, 10(3), 151–164. DOI: 10.4155/tde-2018-0061DOI: 10.4155/tde-2018-0061.
Lim (2023). Complete guide to all 37 FDA new drug approvals in 2022. Ftloscience.com/complete-guide-fda-new-drug-approvals-2022/, accessed March 24, 2023.
Liu et al (2022). Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. Journal of controlled release : official journal of the Controlled Release Society, 348, 84–94. DOI: 10.1016/j.jconrel.2022.05.043.
Moriarty (2022) Bispecific antibodies and cancer immunotherapy. bioradiations.com/bispecific-antibodies-and-cancer-immunotherapy-122/, accessed March 08, 2023.
Paunovska et al (2022). Drug delivery systems for RNA therapeutics. Nat Rev Genet. 23, 265–280 DOI:10.1038/s41576-021-00439-4.
Pharma R&D Annual Review (2022). pages.pharmaintelligence.informa.com/rdreview?utm_source=RDReview2022&utm_medium=whitepaper&utm_id=2296624620
Pratt (2022) Understanding Potency in Cell and Gene Therapy Development. Bio-Rad Bulletin 3373.
Pratt (2022). Advancing gene therapy delivery. Bio-Rad Bulletin 3369.
Statista (2023). Top therapy classes by spending global market 2027 forecast statista.com/statistics/281951/top-therapy-classes-in-developed-pharmaceutical-markets-by-spending/.
Tombácz et al (2021). Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther. 29(11), 3293-3304. DOI:10.1016/j.ymthe.2021.06.004.
Xu et al (2019). Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front Immunol. Frontiers in immunology, 10, 2664. DOI: 10.3389/fimmu.2019.02664DOI: 10.3389/fimmu.2019.02664.
Xue et al (2014). Small RNA combination therapy for lung cancer. Proc Natl Acad Sci U S A. 111(34), E3553-E3561. DOI:10.1073/pnas.1412686111.
BIO-RAD is a trademark of Bio-Rad Laboratories, Inc. All trademarks used herein are the property of their respective owner. © Bio-Rad laboratories, Inc.
Content You'll Also Find Interesting
Recommended Features
-
Image

Meet the New Wave of Therapeutic Antibodies
We look into how new modalities are paving the way for better antibody-based therapies and the potential headaches they bring with them.
-
Image

Antibody Discovery – What the Future Holds
We assess the trends and painpoints in therapeutic antibody discovery and look to the future. Take a look at our predictions.
-
Image

Overcoming Monoclonal Antibody Purification Challenges
Learn critical success factors for monoclonal antibody purification and strategies you can leverage to accelerate production.
Subscribe
Get Topic-Specific Insights
Sign up to stay informed on the latest insights and trends in your field. Receive biweekly articles, news, and more.
