
Western blotting, the transfer of proteins to solid-phase membrane supports, is a powerful and popular technique for the visualization and identification of proteins. After separation by electrophoresis, proteins can be bound to membranes where they are fixed and readily accessible for immunological or biochemical analyses, quantitative staining, or the identification of protein-protein and protein-ligand interactions. This section provides an overview of the methods and workflow of western blotting using Bio-Rad's Stain-Free Western Blotting Workflow for faster separation, transfer, and analysis of proteins.
Introducing Stain-Free Western Blotting
This video will show you how Stain-Free Western Blotting enables you to streamline your blotting experiments to achieve faster and better results.
Page Contents

1. Protein Separation — Electrophoresis
Bio-Rad offers TGX™ (Tris/glycine extended) and TGX Stain-Free™ Precast Gels for protein electrophoresis. The unique TGX gel formulation enables rapid separation of proteins at a high voltage while retaining Laemmli-like separation characteristics using standard sample and Tris/glycine running buffers. Typical sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) conditions for midi gels are 200 V for 30–45 minutes. By comparison, complete electrophoretic separation can be performed at 300 V in 20 minutes with midi-format TGX gels.

2. Protein Visualization
SDS-PAGE followed by Coomassie staining is a standard, widely used method to visualize and confirm protein separation in the gel. However, this involves time-consuming staining and destaining procedures. Another common method is the use of prestained standards, which does not give a complete picture of protein separation.
TGX Stain-Free Precast Gels contain unique compounds formulated into the gel chemistry that allow immediate visualization of proteins across the whole gel without staining. This stain-free technology is exclusive to Bio-Rad and is based on proprietary trihalo compounds. When activated by UV light, these compounds covalently bind to tryptophan residues in proteins and emit a fluorescent signal that is easily detectable with stain-free enabled systems such as Bio-Rad's ChemiDoc Touch, ChemiDoc MP, and Gel Doc EZ Imaging Systems.

3. Protein Transfer (Western Blotting)
After protein separation is verified, the transfer of proteins to the western blot membrane is most commonly performed by electrophoretic transfer. Protocols utilizing standard wet or tank blotting systems typically require transfer times of one hour to overnight for a broad range of protein molecular weights. Traditional semi-dry transfer systems, well suited for proteins ranging from 10–100 kD, can perform transfers in 15–60 min. Bio-Rad's Trans-Blot® Turbo™ System reduces transfer protocols to as little as 3 min while maintaining high-efficiency protein transfer across a wide range of molecular weights.

4. Verification of Protein Transfer
After proteins have been transferred, the transfer efficiency should be checked visually. Again, prestained standards can be used for visualization. However, this method confirms only the transfer of the standards and does not provide a definitive confirmation for all the proteins on the western blot. A better method is to stain the membrane with a total protein stain that allows for the visualization of all proteins on the western blot and also allows the determination of transfer efficiency, molecular weight, relative quantities, and other properties of the transferred proteins.
Post-transfer staining is most commonly performed with Ponceau S, but this method involves time-consuming staining and destaining procedures and potentially introduces errors into western blotting results. Alternatively, Bio-Rad's stain-free technology together with stain-free enabled imaging systems allows for instant verification of protein transfer in addition to increased sensitivity over Ponceau staining. Due to the covalently bound compounds, proteins retain their fluorescence after activation and separation, thus providing the ability to verify protein transfer without any additional processing.
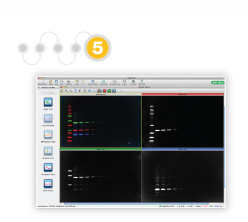
5. Western Blotting Validation and Quantitation
To validate and quantitate proteins in western blotting, the blotted proteins are detected using secondary antibodies, commonly conjugated to fluorophores (fluorescent molecules) or an enzyme such as alkaline phosphatase (AP) or horseradish peroxidase (HRP) that is used to generate a chemiluminescent signal. Use Clarity™ Western ECL Substrate for long signal duration. Less common labeling methods include the use of radioisotopes or colloidal gold.
The ChemiDoc™ Touch Imager can perform both chemiluminescence and multiplex fluorescence detection. Additionally, the stain-free technology can be used for total protein normalization in chemiluminescent western blotting. Bio-Rad’s Image Lab™ Software ensures that acquisition and analysis of the blot are intuitive and flexible through features such as automatic normalization and lane and band detection.
Thus, with five straightforward steps, the Stain-Free Western Blotting Workflow provides a streamlined, more efficient protocol for western blotting.
Videos
This instructional video highlights the main features of the Trans-Blot Turbo system and its easy assembly and transfer process. Helpful tips are provided along the way to ensure successful, rapid, high-quality western blotting.
Documents
TEST
| 6359 | Avoiding Housekeeping Protein Detection Saturation Protocol, Rev A | Click to download |
| 6361 | Determining the Appropriate Film Exposure Time Protocol, Rev A | Click to download |
| 6362 | Determining the Appropriate Sample Load for Western Blots Protocol, Rev A | Click to download |
| 6363 | Determining the Appropriate Sample Load When Using a Stain-Free V3 Western Workflow™ Protocol, Rev A | Click to download |
| 6366 | Validating the Expression Consistency of a Housekeeping Protein Protocol, Rev A | Click to download |
| 6376 | General Protocol for Western Blotting Protocol, Rev A | Click to download |



