Immunodetection (immunological detection) is used to identify specific proteins blotted to membranes. This section provides an overview of immunodetection methods, workflow, protocol, and troubleshooting tips.
Related Topics: Total Protein Detection, Imaging and Analysis.
Page Contents
The steps used for immunological detection vary little and are summarized in the western blotting workflow below.
After the proteins have been transferred to the membrane, the membrane is blocked, incubated with a primary antibody, washed, incubated with a secondary antibody, and washed again. The primary antibody is specific for the protein of interest and the secondary antibody enables its detection. The secondary antibody can be radiolabeled, labeled with a fluorescent compound or gold particles, or conjugated to an enzyme such as alkaline phosphatase (AP) or horseradish peroxidase (HRP) for subsequent detection.
For many years, radiolabeled secondary antibodies were the method of choice for detection, but newer detection reagents have enabled less hazardous and more user-friendly methodologies, while maintaining the same degree of sensitivity. Available detection methods now also include colorimetric, chemiluminescence, fluorescence, bioluminescence, chemifluorescence, and immunogold detection.
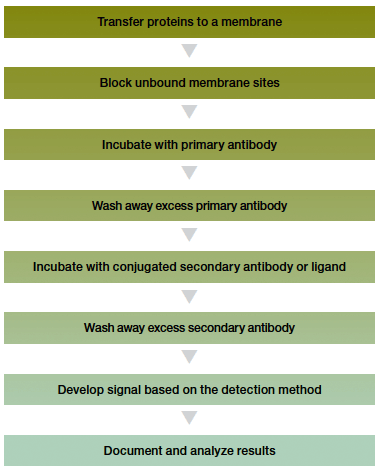
Western blotting workflow.
Following transfer, unoccupied binding sites on the membranes must be blocked to prevent nonspecific binding of probes; failure to completely block these sites can lead to high background. A variety of blocking reagents are available, including gelatin, nonfat milk, and bovine serum albumin (BSA), which are compared in the table below. The detection system can be optimized for minimal background with no loss of signal by testing several reagents. The type of membrane also affects the selection of the blocker.
Comparison of blocking reagents.
| Blocking Reagent | Membrane Compatibility | Recommended Concentration | Notes |
| Gelatin | Nitrocellulose | 1–3% | Requires heat to solubilize |
| Nonfat dry milk, BLOTTO, blotting-grade blocker | Nitrocellulose, PVDF | 0.5–5% | PVDF requires higher concentrations of nonfat milk than nitrocellulose |
| Bovine serum albumin (BSA) | Nitrocellulose, PVDF | 1–5% | PVDF requires higher concentrations of BSA than nitrocellulose |
| Tween 20 | Nitrocellulose | 0.05–0.3% | May strip some proteins from the blot |
A typical immunodetection experimental system utilizes two rounds of antibody incubation:
- The primary antibody, which is directed against the target antigen; the antigen may be a ligand on a protein, the protein itself, a specific epitope on a protein, or a carbohydrate group
- The secondary antibody, which recognizes and binds to the primary antibody; it is usually conjugated to an enzyme such as AP or HRP, and an enzyme-substrate reaction is part of the detection process (see figure below)
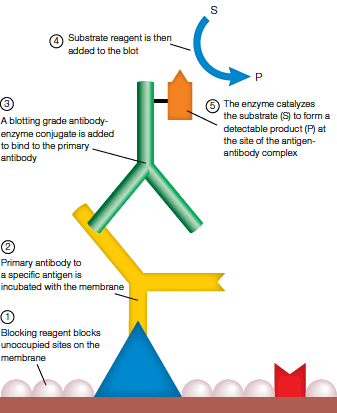
Specific enzymatic detection of membrane-bound antigens.
Antibody incubations are generally carried out in antibody buffer containing Tris-buffered saline with Tween (TTBS) and a blocking reagent.
Washing the blot between the two antibody incubations and prior to detection removes excess antibody and prevents nonspecific binding. Although washing solutions and times may vary (depending on the antibodies and detection systems used), washes generally involve Tris-buffered saline (TBS) or phosphate-buffered saline (PBS). A detergent such as Tween 20 may be added to decrease background, but it may also adversely inhibit certain detection reactions (see the instruction manuals for details).
Immunodetection
| Problem | Cause | Solution |
| Overall high background | Blocking was incomplete |
|
| Blocker was impure. Nonfat dry milk was not pure. The blocker may be contaminated with material that nonspecifically binds probes |
|
|
| Wash protocols were insufficient |
|
|
| The blot was left in the enzyme substrate too long (colorimetric detection) |
|
|
| Contamination occurred during electrophoresis or transfer |
|
|
| Excessive amounts of protein were loaded on the gel or too much SDS was used in the transfer buffer. Proteins can pass through the membrane without binding and recirculate through a tank blotting system |
|
|
| The primary or secondary antibody was too concentrated |
|
|
| Incubation trays were contaminated |
|
|
| Nonspecific reactions between bound proteins and probes | The primary or secondary antibody was contaminated with nonspecific IgG or with IgG cross-reactivity among species |
|
| Monoclonal antibodies reacted nonspecifically with SDS-denatured proteins |
|
|
| Nonspecific interactions are occurring due to ionic associations. For example, avidin, a glycosylated protein, may bind to more acidic proteins on blots |
|
|
| No reaction or weak signal | The sample load was insufficient |
|
| The detection system was not working or is not sensitive enough |
|
|
| Proteins were washed from the membrane during assays |
|
|
| Antigen binding to the membrane was insufficient |
|
|
| Antigen denaturation occurred during electrophoresis or transfer |
|
|
| Epitope was blocked by total protein stain |
|
|
| The primary or secondary antibody was inactive or nonsaturating |
|
|
| The enzyme conjugate was inactive or nonsaturating |
|
|
| The color development reagent was inactive |
|
|
| Regions of poor or uneven signal during detection | The membrane was allowed to dry during handling |
|
* Tests for Monitoring Reagent Activity
- Test the activity of the color development solution. Combine 1.0 ml of the color development solution with 10 µl of full-strength secondary antibody conjugate. The color reaction should occur immediately. If color fails to develop within a few minutes, the color development solution is inactive. Prepare a fresh working solution and repeat the color development assay.
- Test the activity of the conjugate solution. Combine 1.0 ml of the color development solution tested above and 1.0 ml of the 1:3,000 dilution conjugate solution. A light-blue tinge should develop within 15 min. If color fails to develop within 25 min, the conjugate solution is suspect. Repeat the procedure with a freshly prepared dilution of conjugate.
- Test the activity of the first antibody solution. Use an ELISA, RID, Ouchterlony immunodiffusion, or precipitation test to determine reactivity of the antibody with the antigen. If possible, repeat the assay procedure with a more concentrated primary antibody solution.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6216 | Detection Buffer Formulations | Click to download |
| 6218 | Blot Stripping & Reprobing | Click to download |
| 6219 | Immunodetection | Click to download |
