The hallmark of stem cells is their ability to self-renew and to differentiate into different cell types. The induction of stem cell differentiation into a given cell type often requires a specific combination of media and factors. This section provides a brief overview of methods for differentiating human embryonic stem cells (hESCs) into different cell types.
Related Topics: Stem Cell Research, Isolation and Maintenance of Stem Cells, Transfection and Transduction of Stem Cells, Analysis of Stem Cells, and Stem Cells in Therapeutics and Research.
Page Contents
As with culturing stem cells, methods of differentiation depend on the type of stem cell, the species, target lineages, and somatic cell types. When stem cells are being induced to differentiate, it is essential that the progress be tracked and that the phenotype of the cells be confirmed. The lineages and identities of differentiated cell types can be analyzed using PCR techniques such as real-time PCR or digital PCR, cell sorting/flow cytometry, immunocytochemistry, western blotting, and biomarker analysis.

ESCs and induced pluripotent stem cells (iPSC) can form embryoid bodies, which can differentiate into cells of the ectoderm, mesoderm, and endoderm.
One of the oldest methods for stem cell differentiation is the generation of embryoid bodies (EBs). Generally, when stem cells are cultured without an adherent surface, feeder cells, or a complex matrix, the cells aggregate. These aggregated cells spontaneously differentiate. An EB contains all three germ layers.
EBs are still often used as the initial stage of differentiation for embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). All downstream differentiated cells are derived from this initial structure. There are several methods for forming EBs, including suspension culture, hanging-drop culture, and culture in semisolid media. All protocols start with detaching a high-density cell culture from the dish, either enzymatically or with versine, depending on the properties of the cells.
Suspension culture is the most common method for EB formation but also the hardest to control, for both EB size and shape; EBs can become large and irregularly shaped in suspension. Suspension culture is scalable to bioreactors, although the exact methods and factors such as stir rate that affect the physical dimensions of EBs must be determined empirically.
Hanging-drop culture gives smaller and much more uniform EBs. The size of the EBs can be controlled by controlling the number of cells in each drop. Making the drops (usually ~20 μl) was formally labor intensive, and hence the numbers of EBs generated was low. Now, higher-throughput hanging-drop methods using arrays have been developed and used for anticancer-drug sensitivity testing (Hsaio et al. 2012).
Semisolid media, usually methylcellulose, can be used to suspend the EBs. This method is efficient for making EBs, but is not as scalable as suspension culture.
ESCs form EBs in about four days, and are often allowed to grow for weeks. All three germ layers are present, but the composition of the media influences the ratios of cell types and lineages.

Embryoid bodies after growing in suspension for eight days. Image courtesy of Dr. Miguel Esteban.
Depending on the desired end point, the presence of all three germ layers in EBs can be a disadvantage. When a specific lineage or cell type is required, cultures must be depleted of the unwanted cell types. Therefore, many protocols have been developed for directly differentiating ESCs and iPSCs without using EBs. The methods are specific for the species, lineage, and cell type.
The central nervous system, hair, and the epidermis are all derived from the ectoderm. There are several protocols for producing neural progenitor cells from undifferentiated cultures. One of these protocols is described below (Zhang et al. 2001). This protocol has been the basis for the generation of a number of different neuronal cell types.
To induce EBs to form neurons, the culture medium is replaced with neural basal media containing bFGF (basic fibroblast growth factor) heparin, and N2 supplement. N2 supplement consists of transferrin, insulin, progesterone, putrescine, and selenite. Two days later, attachment of the differentiating EBs is induced by plating them onto dishes coated with laminin or polyornithine. After an additional 10–11 days in culture, the EBs differentiate into primitive neuroepithelial cells. The identity of the cells can be confirmed by staining for PAX6 (paired box protein 6, a transcription factor), SOX2 (sex-determining region Y-box 2, another transcription factor), and N-cadherin (a calcium-dependent cell adhesion molecule specific to neural tissue).
At this point, it is possible to differentiate the neuroepithelial cells into specific cell types of the central nervous system including motor neurons (Li et al. 2005), dopaminergic neurons (Yan et al. 2005), and oligodendrocytes (Nistor et al. 2005).
Cells of the mesoderm form most of the body's internal supporting structures, including blood, muscle, bone, cartilage, and heart. Because new mesodermal cells have potential for use in the treatment of common ailments such as osteoarthritis, osteoporosis, and cardiovascular disease, there has been a major focus on protocols for stem cell differentiation into mesodermal cells.
Cardiomyocytes develop spontaneously from 10-day-old EBs plated onto gelatin-coated plates (Kehat et al. 2001). Fortunately, although they are a small percentage of the cells, cardiomyocytes are easy to identify due to their hallmark rhythmic contractions. Cardiomyocytes can be separated from the rest of the differentiating culture by cell sorting using antibodies against cardiac markers (Xu et al. 2002).
An alternative method for deriving cardiomyocytes is to transfect a stem cell culture with a viral vector containing a drug-resistance gene driven by the alpha-myosin heavy chain promoter. Subsequent selection for drug resistance enables the selection of cells that differentiate into cardiomyocytes (Zhao and Lever 2007).
The differentiation of stem cells into cells within the hematopoietic lineage has long been of important clinical interest for cancers of the blood, such as leukemia. Early work led to the development of techniques for differentiating human embryonic stem cells (hESCs) cells into most of the cells of the hematopoietic system (Keller et al. 1993, Kaufman et al. 2001). More recently , the potential for creating replacement cells for blood transfusion, blood cell disease, vascular disease (with endothelial cells), and immunodeficiency disorders has increased interest in the development of techniques for differentiating both iPSCs and hESC into cells of the hematopoietic and vascular systems, and clinical trials have been undertaken for stem cell-derived therapies for leukemia, lymphoma, and sickle cell disease (Nature Biotechnology News 2014).
The endoderm forms many of the internal organs, including the pancreas and the liver. High rates of diabetes and liver disease have made the production of insulin-secreting cells and hepatocytes key goals in the field of stem cell research.
Type 1 diabetes is caused by the destruction of the insulin-secreting beta cells of the Islet of Langerhans in the pancreas. Current treatment options include pancreatic transplants or the infusion of donor beta cells. However, donors are in short supply, and beta cell transplantation is usually not a permanent cure due to immune response in the recipient, leading to destruction of the donated beta cells.
It is now possible to make human embryonic stem cells into all pancreatic cell lineages (Guo and Hebrok 2009). However, the beta-like cells produced during the complex differentiation process are not efficient insulin producers and are not as completely responsive to cell signaling as native beta cells (Furth and Atala 2009). A breakthrough in this line of research was recently reported in which large numbers of functional human pancreatic β cells were generated in vitro, providing an unprecedented cell source for drug discovery and cell transplantation therapy in diabetes (Pagliuca et al. 2014). Progress toward developing liver cells (hepatocytes) for transplantation has been slow. Stem cells have been differentiated into hepatocyte-like cells using several methods (Hay et al. 2008, Basma et al. 2009, Szkolnika et al. 2014) but are generally not suitable for transplantation.
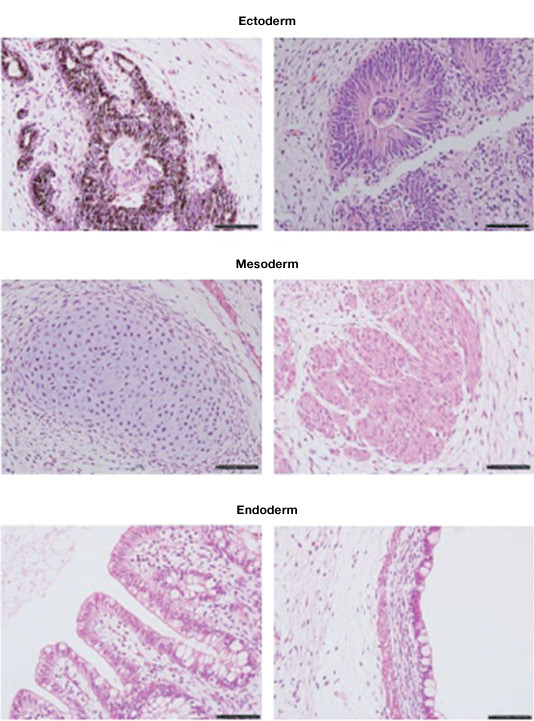
Teratomas composed of tissues derived from the three germ layers produced after injection of an ESC cell line into immunosuppressed mice. Image courtesy of Dr. Miguel Esteban.
The ability of stem cells to differentiate into nearly any cell in the body gives them the potential to form the basis of therapies for many conditions. As research moves forward, standardized techniques for stem cell culture and differentiation will be developed. These new techniques will lay the foundation for research into cells and tissues and future stem cell therapies.
Basma H et al. (2009). Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136, 990–999. PMID: 19026649
Furth ME and Atala A (2009). Stem cell sources to treat diabetes. J Cell Biochem 106, 507–511. PMID: 19130494
Guo T and Hebrok M (2009). Stem cells to pancreatic beta-cells: New sources for diabetes cell therapy. Endocr Rev 30, 214–227. PMID: 19389995
Hay DC et al. (2008). Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26, 894–902. PMID: 18238852
Hsaio AY et al. (2012). 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechol Bioeng 109, 1293–1304. PMID: 22161651
Kaufman DS et al. (2001). Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 98, 10716–10721. PMID: 11535826
Kehat I et al. (2001). Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108, 407–414. PMID: 11489934
Keller G et al. (1993). Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol 13, 473–486. PMID: 8417345
Li XJ et al. (2005). Specification of motor neurons from human embryonic stem cells. Nat Biotechnol 23, 215–221. PMID: 15685164
Nature Biotechnology News (2014). Novartis wades into stem cell therapies. Nat Biotech 32, 969. doi:10.1038/nbt1014-969e
Nistor GI et al. (2005). Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385–396. PMID: 15538751
Pagliuca FW et al. (2014). Generation of Functional Human Pancreatic β Cells In Vitro. Cell 159, 428–439. PMID: 25303535
Szkolnika D et al. (2014). Deriving functional hepatocytes from pluripotent stem cell. Curr Protoc Stem Cell Biol. 30, online ahead of print PMID: 25081564
Xu C et al. (2002). Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 91, 501–508. PMID: 12242268
Yan Y et al. (2005). Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790. PMID: 15917474
Zhao J and Lever AM (2007). Lentivirus-mediated gene expression. Methods Mol Biol 366, 343–355. PMID: 17568135
Zhang SC et al. (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19, 1129–1133. PMID: 11731781
