Even though stem cell research has grown leaps and bounds in the last two decades, the handling of stem cells still require well tested protocols and instrumentation made with stem cells in mind. The need for sorting stem cells is universal across different types of stem cell research. Capturing stem cells in their various states of being, while keeping them pluripotent and healthy, is no trivial task. While several methods for sorting one cell type from another exist, most methods have significant limitations and are only able to help with a narrow scope of stem cell research.
In contrast, fluorescence-activated cell sorting is a highly flexible technique with a large range of stem cell sorting capabilities. It is also the only way to isolate a highly specific stem cell population using multiple internal and external markers. Cell sorting is fast, for isolation of rare stem cell populations, and very precise for research requiring ultra-high purity of a specific stem cell population. As the field of stem cell research grows, so do the types of stem cells investigated, and genetic manipulation techniques used. Fluorescence-activated cell sorting provides the flexibility for the current and future cell isolation needs for efficient stem cell research.
Page Contents
FACS is a specialized type of flow cytometry that sorts a heterogeneous mixture of cells based upon the specific light scattering and fluorescent characteristics of each cell. Cells are first tagged using fluorescent antibodies that bind to relevant proteins on target cells. Expression of fluorescent proteins or intercalating dyes can also be used to label cells before sorting. The mixture of suspended cells rapidly flows through the instrument's flow cell in a single file. Individual cells pass a laser beam, and a detector measures the fluorescence, forward-scattered light (FSC), and side-scattered light (SSC). FSC and SSC are used to determine the cell’s size. Fluorescence at several wavelengths can be detected simultaneously but depends on the instrument’s specific lasers, filters, and detectors. Cells are then physically sorted into different containers based on detected measurements and user entered parameters.
For experiments where highly pure populations of stem cells need to be isolated, FACS provides a method that achieves high purity, viability, and throughput.
- Purity — experimentation and clinical therapies that use stem cells require a high purity of target cells for accurate analyses and patient safety. Cell sorting is often the only way to achieve ultra-high purity of stem cells, often reaching purities over 99%
- Viability and vitality — all stem cell samples are precious due to their rarity and difficulty in culturing. Isolation techniques need to keep cells viable and preserve vitality. Cell sorting using flow cytometry can achieve highly pure homogeneous samples where 99% of the cells are viable and functional
- High-throughput — somatic stem cells, CSCs, iPSCs, and CRISPR edited stem cells all exist at very low concentrations within a population of cells. Isolation of these types of target cells needs to be performed quickly due to the fact that stem cells require cell-to-cell interactions and are grown in aggregates or colonies to thrive. Cell sorting using modern instrumentation is very high-throughput with some benchtop systems even able to reach up to 10,000 events/second and therefore ensuring that enough cells can be collected for downstream applications and experimentation
- Multi-parameter isolation — isolation of stem cells, progenitor cells, or differentiated cells may require detection of several cellular characteristics to reliably pick out target cells. Fluorescence activated cell sorting has the ability to detect multiple attributes of a cell simultaneously for efficient and dependable target cell isolation. Surface marker staining, internal fluorescent proteins, and DNA dyes can all be used to label target cells. FSC and SSC determine cell size and shape. With the ability to sort using multiple parameters, a very specific population of cells can be reliably identified and isolated

A fluorescence-activated cell sorter provides the ability to separate cells identified based on size and/or fluorescence. Droplet based cell sorters first analyze the particles, but also have hardware that can generate droplets and a means of deflecting or directing wanted particles into a collection tube. Droplets can be formed by using high-frequency (cycles/second, Hz) vibration of the nozzle at an optimal amplitude (in volts) over a period of time. This is typically created by a piezoelectric crystal. As cells pass through the laser interrogation point, light emitted from the cell is collected via detectors, and this signal is then processed by the instruments electronics which then tells the instrument to apply a charge to the droplet. The charged droplet can then be deflected into the appropriate tube for collection.
Challenges of Stem Cell Isolation Solved Using FACSEvery stem cell population is unique in its physical properties and expression patterns making the challenges of stem cell isolation different for various applications. The most flexible technique available for stem cell isolation is FACS. In fact, it is the only technique that can sort cells based on an internal fluorescent marker, or degree of expression of multiple surface proteins.
To prevent unwanted differentiation, stem cells need to be handled with care and cultured within a population. Modern cell sorters are fast and gentle on stem cells to ensure their survival and pluripotency. When differentiation is what’s wanted, current sorting technology allows for over 99% purity of differentiated cells to avoid detrimental stem cell contamination in pre-clinical and clinical research. Purity is also critical when genetically editing stem cells for functional research. Across the board, FACS is a technique that solves many of the challenges associated with stem cell isolation through fast, flexible, and gentle sorting.
Somatic Stem Cells (SSCs) have become a main source of current stem cell research due to their availability in all humans, and non-controversial isolation. As the field grows, new techniques to manipulate SSCs are being developed to make the cells more clinically useful. While SSCs in regenerative and tissue replacement therapies are extremely promising, their isolation from various tissues poses special challenges due to their rarity and need for purity in clinical experimentation.
Rare and Diverse Tissue SSC PopulationsDepending on the tissue of origin, desired SSCs can exist in very low population frequencies. For example, CD34+ hematopoietic stem cells (HSCs) make up only 0.1–0.2% of peripheral, on average. In terms of cell frequency, this translates into approximately 1000–7000 cells per mL of blood. Depending on the downstream application, it can be extremely challenging to collect enough cells for analysis.
However, isolation of such a rare cell population becomes possible when using the high-throughput capabilities of FACS. This was illustrated in a recent study that used cell sorting to isolate CD34+ and CD45- cells from diverse mouse tissues (Shenoy, et al.). The presence of mesenchymal stem cells in mouse skin, muscle, and liver was verified, and isolated cell populations were large enough for downstream characterization using mass spectrometry.
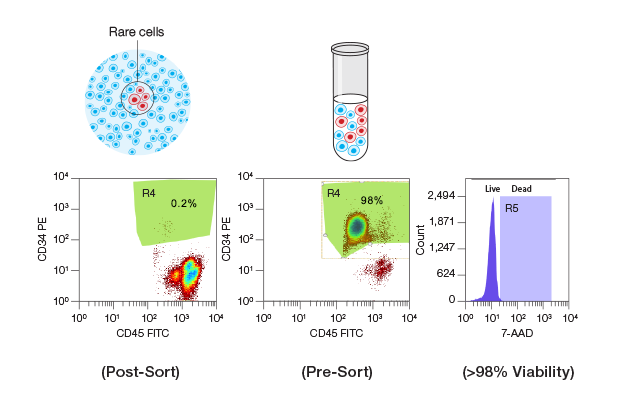
FACS enables the high-speed isolation of rare and low-abundance cell populations. Human CD34+/CD45+ hematopoietic stem and progenitor cells (HSPCs) with a population frequency of 0.2% (green gated area in the right dot plot graph) were isolated from peripheral blood mononuclear cells (PBMCs) at 10,000 events per second on the S3e Cell Sorter. Post-sort purity analysis demonstrated that this population was enriched to 98% purity (green area on the right dot plot) and >98% viability (purple peak on the far right graph).
Isolating Mesenchymal and Muscle Stem CellsThe ability to efficiently isolate stem cells from diverse tissues is critical not only for cell characterization, but also for use in pre-clinical research and therapeutics (e.g. tissue replacement and regenerative cell therapy). Mesenchymal stem cells (MSCs) derived from bone marrow, and muscle stem cells (MuSCs) have proven themselves an important source material for these types of therapies. MSCs are multipoint stromal cells, non-hematopoietic in nature, and have the potential to differentiate into diverse cell types (Potdar et al.). This property is exploited to research treatment for a wide variety of diseases including neurodegenerative, auto-immune, and cardiovascular diseases. Cartilage regeneration is also a promising therapy currently being researched using MSCs isolated from bone marrow using cell sorting instrumentation (Beane, et al.). MSCs can be labeled with cell surface markers allowing sorting based on fluorescence.
MuSCs, also known as satellite cells, normally proliferate in response to muscle injury and are highly promising in use for treatments of muscular degenerative diseases or traumatic injury. To isolate MuSCs, a cocktail of antibodies are used to stain various markers and cell sorting is used to differentiate MuSCs from non-target cells. Staining of the precise markers that define MuSCs from other cells is critical to their effective isolation and has been improved over time to yield even more precise populations of quiescent and active MuSCs (Liu, et al.).
 |
Sorting Bone Marrow Mesenchymal Stem Cells |
For use in pre-clinical and clinical research, SSCs need to be isolated with very high purity and viability, which can pose another technical challenge. In addition, some of the more applicable stem cell populations are only distinguishable from undesired cell types by the concentration of a defining marker, e.g. high vs. low expression or bright vs. dim fluorescence intensity (Gori, et al.). For example, a recent study found a subpopulation of cells within bone marrow with the ability to differentiate toward the mesenchymal lineage, while still retaining angiogenic potential (Pacini, et al). Using multi-color flow cytometry in combination with FACS, this population — named Pop#8 — was characterized as CD64bright, CD31bright, and CD14neg. Once the defining markers were identified, cell sorting was performed on fresh bone marrow using these parameters and the isolated Pop#8 cells were shown to differentiate into MSCs on demand.
For characterization experiments, stem cell purity is important, but in clinical research, in which cells are transplanted into a living being, purity is critical. Contamination with other cell types has been shown to cause undesirable effects in the receiving patient or animal model. Impure cell populations have been identified as a reason for some stem cell transplants to have highly variable clinical benefit (Carlsson, et al.). Autologous stem cell transplantation for the treatment of hematological malignancies, for example, has been used for over two decades with variable engraftment success. When investigating engraftment purity, quality, and CD34+ cell status, a recent study concluded that all three factors contributed significantly to the success of engraftments (Giai, et al.).
In a separate case of consequences from impure stem cell populations, careless contamination of MSCs with human fibrosarcoma cells have led to several erroneous reports of spontaneous MSC transformation (Herberts, et al. ). Seeing as 18 to 36% of all cell lines contain misidentified species, contamination is clearly a problem that cannot be ignored. These kinds of occurrences temporarily lead the field astray and can be avoided with more diligent characterization of desired cells and standard cell sorting procedures.
FACS is the only method of SSC isolation that can yield ultra-high purity samples reaching levels greater than 99% purity. In addition, cell sorting maintains cell viability and sorts at a pace considered high-throughput when isolating rare cell populations. Whether in initial stages of SSC characterization or at the clinical research phase for tissue replacement and transplantation, cell sorting provides a method for efficient and effective SSC isolation.
Recent progress in the field of Induced Pluripotent Stem Cells (iPSCs) has opened up the opportunity to use these cells in clinical research for regenerative medicine and as in vitro models for human disease. iPSCs, like embryonic stem cells (ESCs), possess properties of self-renewal and differentiation to many types of cell lineage. The process of differentiation of iPSCs or ESCs is itself technically challenging and results in imperfect yields. For best results, especially for use in clinical research, ultra-high purity populations of differentiated cells with high viability are needed, but can be difficult to isolate (Andrews, et al.).
Identifying and Isolating Cultured ESCs and iPSCs with a Normal KaryotypeOne of the major technical issues when culturing ESCs or when reprogramming somatic cells to a pluripotent state is ensuring a normal chromosomal karyotype after multiple rounds of passage. Genome instability has plagued pluripotent stem cell culture, resulting in changes in ploidy, chromosome copy number, or nucleotide variants (Popp, et al. and Giulitti, et al.). It has been reported that chromosomal abnormalities are accelerated in pluripotent stem cells when cultured in suboptimal conditions, such as the use of Trypsin for cell dissociation (Theunissen, et al. ). In addition, clones with abnormal karyotypes will often outgrow pluripotent cells that maintain a wild-type karyotype (Liang et al.).
Genetic integrity of these cells is critical for their use and validity as disease models and potential regenerative therapies. Therefore, optimal cell culture practices should be implemented, and cell sorting used to isolate cells with normal karyotypes. DNA stains with low toxicity, made specifically for use in live cells, have been used to isolate genetically stable pluripotent stem cells using FACS.
In one example, naive iPSC cell lines were tested for ploidy over the course of extensive culture (Giulitti, et al.). After the first 8 passages, all cell lines were diploid. After 42 passages, 6 out of the 8 cell lines remained diploid. The other 2 cell lines showed a fraction of tetraploidy after 12 or 23 passages. Using a low toxicity DNA stain with FACS, diploid cells were isolated away from the tetraploid cells, which then maintained a diploid karyotype after further culture.
Purifying Differentiated CellsMany differentiation protocols have low efficiency and often result in residual undifferentiated stem cells. For example, in one GWAS study, differentiation of iPSCs derived from 34 different patients into adipocytes resulted in a mean cumulative efficiency of 60%, while in the same study, differentiation to hepatocyte-like cells had a mean cumulative efficiency of only 13%. In studies that use differentiated cells to research disease association with genetic variants, non-differentiated cells need to be removed from the population in order to properly represent the tissues of interest.
Fluorescent tagging of cell-surface markers combined with cell sorting is a reliable method for isolating differentiated cells from the undifferentiated. In one example, a protocol for the differentiation and isolation of primordial germ cell-like cells (PGCLCs) from pluripotent stem cells (PSCs) was developed, incorporating a cell sorting step (Irie, et al.). After differentiating cells using several chemical agents, they used a gating strategy set to account for their NANOS3-mCherry reporter, TNAP, and CD38 staining. Using FACS, PGCLCs were efficiently separated from PSCs.
In another example, iPSCs were differentiated to iPSC endothelial progenitors (EPs) as a promising candidate cell source for patient-specific ischemic therapies (Crosby, et al.). To account for subpopulation heterogeneity of differentiated iPSC-EPs, CD34 was used as a marker to sort cells into separate populations using with the S3e Cell Sorter. The group found that even though the cells were treated equally during the differentiation protocol, CD34high cells had very different expression profile from CD34low/negative cells. It’s clear that cell sorting is an essential step following PSC differentiation for isolation of differentiated cells only.
If differentiated ESCs or iPSCs are to be used in cell replacement therapies, impure engraftments run the considerable risk of teratoma formation. Teratomas are benign tumors containing various tissues that form when a portion of cells injected into a patient are still pluripotent due to incomplete differentiation. Even a small fraction of undifferentiated cells can cause a teratoma. This risk is one of the most critical barriers for stem cell applications in regenerative therapy for diseases such as Parkinson’s, and cardiac failure (Ambasudhan, et al. and Ban, et al.). Cell sorting has been suggested as a strategy to gain enough purity to lessen this risk. FACS is a way to achieve over 99% purity of target cells and decrease the risk of pluripotent cell contamination.
Fluorescent protein reporters have been invaluable to the advancement of stem cell research. Marking cells after genetic manipulation or to view developmental progression gives us great insight into stem cell fate and contribution to a whole organism. While staining of cell surface markers is very useful for some applications of stem cell isolation, the technique becomes less useful for tracking of engineered stem cells. To isolate cells based on internal fluorescence, FACS is the only technique available.
 |
High-Speed Sorting of Rare Cell Populations with High Purity and Viability Using the S3e Cell Sorter |
The advancement of CRISPR technology has changed the game for genetic research. Used in combination with iPSCs generated from patients with a particular condition, powerful disease models can be generated and tested for the efficacy of novel treatment strategies. Since iPSCs can produce essentially any cell type, specialized cell types relevant to the disease of interest can be generated and CRISPR used to investigate the influence of particular genes on cell or tissue function.
Unfortunately, gene editing of stem cells is notoriously inefficient and stem cells in isolation are unstable. This results in a tiny population of edited stem cells that need to be isolated from non-edited cells very quickly and with high viability. One solution that helps tremendously with the enrichment of edited cells is the addition of a fluorescent reporter, typically a fluorescent protein attached to Cas9. These reporters enable rapid isolation of edited cells using FACS for generation of disease models or testing of genetic factors in disease.
Competent disease modeling not only relies on specific cell types for experimentation, but also characteristic 3D tissue structures and interactions with additional cell types. In one recent study, lung bud organoids (LBOs) were generated from human PSCs showing promise as a model for lung disease (Chen, et al.). After xenotransplantation and in Matrigel 3D culture, the LBOs developed into branching airway and early alveolar structures. Introduction of a mutation in HPS1 led to accumulation of extracellular matrix and mesenchymal cells, suggesting the potential use of this model to recapitulate fibrotic lung disease in vitro. To generate LBOs with the HSP1 mutation, CRISPR technology was used in combination with an mCherry reporter to tag cells that successfully received the electroplated Cas9 protein. FACS was used to isolate cells positive for mCherry for further experimentation with HPS1 mutated LOBs.
In another study, rat ESCs were manipulated using CRISPR technology as a means to develop more disease models using rats (Chen, et al.) Rat ESCs have proven difficult to work with, as they require expert handling to maintain robust growth, especially after clonal selection required for gene targeting, which has hindered their use as a disease model. In this study, rat ESCs were genetically edited using CRISPR through transfection of an expression plasmid containing guide RNA, and GFP-Cas9. Cells were sorted based on GFP fluorescence using FACS with high enough purity and viability to enable in vitro studies and for generating genetically modified rats. The use of this method will enable the development of disease models and genetic experimentation using rats, which in some cases prove a more viable solution than using mice.
Lineage Tracing and Single-Cell AnalysisCell differentiation, while now used as a technique to guide stem cells to become various tissues, is a naturally occurring phenomenon within living organisms that gives rise to a variety of cell types. Lineage tracing is an investigative approach that enables researchers to dissect the developmental trajectories of stem cell subsets and utilizes fluorescent reporters to allow researchers to isolate various populations after organismal dissection. Further, tracing cell lineage from an embryonic state allows for more comprehensive characterization of the many different cells, microenvironments, and networks.
Today, researchers are now combining powerful single-cell analysis applications with lineage tracing to help uncover hidden cell populations that give rise to different cell types. In one study, a fibroblastic reticular cell (FRC)-specific fate-mapping in mice was established to define the embryonic origin and differentiation trajectories of splenic white pulp mesenchymal lymphoid tissue organizer (mLTo) cells (Cheng, et. al.). FACS was used to sort splenal fibroblastic stromal cells expressing an EYFP reporter indicating cells of a specific origin. Sorted cells were then processed for single-cell RNA-sequencing (scRNA-seq) to reveal distinct expression signatures. By combining in vivo cell-fate mapping and scRNA-seq, differentiation trajectories of white pulp reticular cell networks were uncovered and mLTo cells comprehensively characterized.
Fluorescent Nuclei IsolationExperimentation requiring the isolation of a subset from a population of nuclei poses a significant challenge, as nuclei do not exhibit typical cell surface markers. Instead, fluorescent markers inside nuclei can be used as differentiating factors isolated using FACS. In fact, FACS is the only way that a subpopulation of cells or nuclei can be isolated with internally expressed markers.
Isolating nuclei that express fluorescent proteins helps to perform analyses to understand genomic mechanisms driving stem cell function. In a recent study, for example, nuclei from drosophila neural stem cells were isolated to enrich for chromatin before analysis by chromatin immunoprecipitation (ChIP) (Arya, et al.). dsRed was used as a reporter to mark cells of the central nervous system for sorting from other cells of the drosophila embryo. FACS was used to isolate neural stem cell nuclei in drosophila with, or without knock-down of the cut gene. ChIP and qPCR analyses of the sorted nuclei uncovered dependence of chromatin conformation on cut, and revealed the mechanism by which cut regulates neural stem cell apoptosis.
Although not classified under the classical stem cell umbrella, cancer stem cells (CSCs) possess characteristics of normal stem cells such as self-renewal and differentiation into different cell types. CSCs are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. In order to develop more effective therapies against cancers with CSCs, we need to understand their unique biology.
Promising CSC Therapeutic ResearchResearch in the field is progressing. In one study, it was found that CD47 was highly expressed on glioma cells, especially glioma stem cells (GSCs), and that expression associated with worse clinical outcomes (Li, et al.). CD47 acts as an anti-phagocytic “don't eat me” signal and is often found expressed by cancer cells, particularly CSCs. Treatment with anti-CD47 antibodies in vitro and in vivo resulted in increased phagocytosis of glioma cells and GSCs, inhibition of tumor growth, and prolonged survival times. While this research is a promising step in the direction of novel CSC targeting therapies, it is only the beginning. Additional characterization of CSCs needs to be performed, and the first step in that process is isolation from tumors.
In fact, more recent research dove deeper into the mechanism behind the CD47 immune blockade. SIRPα, a membrane glycoprotein found on macrophages binds to CD47 and signals ‘self’ to inhibit phagocytosis. In a genetic screen, cells were sorted using FACS through conjugation of an antibody that bound to the SIRPα recognition site of CD47 (Logtenberg, et al.). Using this method, glutaminyl-peptide cyclotransferase-like protein (QPCTL) was identified as a major component of the CD47-SIRPα checkpoint. QPCTL was demonstrated to be critical for pyroglutamate formation on CD47 at the SIRPα binding site shortly after biosynthesis, and presents QPCTL as a potential novel target for cancer therapy.
Identifying and Isolating Cancer Stem Cells from Various TissuesThe isolation of CSCs from a tissue biopsy carries unique challenges. Patient samples can be difficult to obtain, and are limited in volume and frequency. Precious samples require an isolation technique with a high recovery of target cell subsets in order to avoid waste and have enough cells for downstream assays. In addition, biopsy quality, in terms of cell quality and viability, will vary from patient to patient. Biopsies will also vary in the number of CSCs present, with the literature reporting as little as 0.0001%, and as much as 40%. In times when CSCs are very rare, the isolation technique is critical for capturing as many CSCs as possible while keeping the population pure and highly viable.
Another consideration is that CSC isolation needs to be precise, which requires the use of multiple markers and instrumentation able to accurately sort target cells from the rest of the population. For example, separating CSCs from cancer progenitor cells (PGCs) calls for the use of several cell surface and internal cell markers. In a recent study, researchers showed that the use of CD44 and CD133 as markers, typically associated with stem cells, were not enough to separate CSCs from PGCs in murine gastric cancer samples. The addition of aldehyde dehydrogenase (ALDH) activity as an additional marker allowed the researchers to identify and separate CSCs from PGCs. FACS is the only method that can separate cells based on multiple markers and can provide the flexibility for this kind of research.
In the past, cost and inconvenience have been barriers to access of FACS instrumentation. Often, cell sorters are located in specialized, difficult to access facilities. Older instrumentation can also be technically complex and difficult to learn how to use, requiring dedicated personnel for operation and maintenance, which greatly limited access. In some cases, instrument setup would be too harsh on cells and unsuitable for isolating fragile stem cells. Fortunately, modern cell sorters are more accessible in all ways. Lower cost, easier setup, and advancements in multi-parameter sorting technology make FACS a safe bet for all stem cell isolation needs.
Interested in using FACS? Bio-Rad's S3e Cell Sorter enables simple sorts or isolation of low-abundance cell types right in your own lab. With features that simplify the complex and error-prone aspect of cell sorting, the S3e demonstrates that it's possible for an automated cell sorter to provide exceptional sort purity and viability without compromising performance or sensitivity.
Check out the S3e Cell Sorter!
Ambasudhan R et al. (2014). Potential for cell therapy in Parkinson"s disease using genetically programmed human embryonic stem cell-derived neural progenitor cells. J Comp Neurol 522(12):2845-56 PMID: 24756727
Andrews PW et al. (2017). Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. Stem Cell Reports 9(1):1-4 PMID: 28700896
Ban K, Bae S, Yoon YS (2017). Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics 7(7):2067-2077 PMID: 28638487
Beane OS, Darling EM (2012). Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann Biomed Eng 40(10):2079-97 PMID: 22907257
Carlsson T et al. (2007). Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Neurosci 27(30):8011-22 PMID: 17652591
Chen Y et al. (2017). Gene Editing in Rat Embryonic Stem Cells to Produce In Vitro Models and In Vivo Reporters. Stem Cell Reports 9(4):1262-1274 PMID: 29020614
Chen YW et al. (2017). A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19(5):542-549 PMID: 28436965
Cheng HW et al. (2019). Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp. Nat Commun 10(1):1739 PMID: 30988302
Crosby CO et al. (2019). Quantifying the Vasculogenic Potential of Induced Pluripotent Stem Cell-Derived Endothelial Progenitors in Collagen Hydrogels. Tissue Eng Part A 25(9-10):746-758 PMID: 30618333
Giai V et al. (2016). Graft Purity and Composition Significantly Impact the Engraftment of Autologous Stem Cell Transplants. Blood 128 (22): 5733
Giulitti S et al. (2019). Direct generation of human naive induced pluripotent stem cells from somatic cells in microfluidics. Nat Cell Biol 21(2):275-286 PMID: 30598530
Gori JL et al. (2012). Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells. Blood 120(13):e35-44 PMID: 22898598
Herberts CA, Kwa MS, Hermsen HP (2011). Risk factors in the development of stem cell therapy. J Transl Med 0.395138888888889 PMID: 21418664
Irie N, Surani MA (2017). Efficient Induction and Isolation of Human Primordial Germ Cell-Like Cells from Competent Human Pluripotent Stem Cells. Methods Mol Biol 1463:217-226 PMID: 27734359
Li F et al. (2018). Blocking the CD47-SIRPα axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 7(2):e1391973 PMID: 29308321
Liang G, Zhang Y. (2013). Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell 13(2):149-59 PMID: 23910082
Liu L, Cheung TH, Charville GW, Rando TA. (2015). Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc 10(10):1612-24 PMID: 26401916
Logtenberg MEW et al. (2019). Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat Med 25(4):612-619 PMID: 30833751
Pacini S et al. (2016). Mesangiogenic Progenitor Cells Derived from One Novel CD64(bright)CD31(bright)CD14(neg) Population in Human Adult Bone Marrow. Stem Cells Dev 25(9):661-73 PMID: 26975798
Popp B et al. (2018). Need for high-resolution Genetic Analysis in iPSC: Results and Lessons from the ForIPS Consortium. Sci Rep 8(1):17201 PMID: 30464253
Potdar PD, Deshpande SS (2013) Mesenchymal Stem Cell Transplantation: New Avenues for Stem Cell Therapies. J Transplant Technol Res 3: 122 doi: 10.4172/2161-0991.1000122
Qiu L et al. (2017). Immature Midbrain Dopaminergic Neurons Derived from Floor-Plate Method Improve Cell Transplantation Therapy Efficacy for Parkinson"s Disease. Stem Cells Transl Med 6(9):1803-1814 PMID: 28650520
Sudheer Shenoy P, Bose B (2017). Identification, isolation, quantification and systems approach towards CD34, a biomarker present in the progenitor/stem cells from diverse lineages. Methods 131:147-156 PMID: 28684339
Theunissen TW et al. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15(4):471-487 PMID: 25090446
