Total protein staining provides an image of the protein migration pattern in a gel or on a blot. This information helps determine transfer efficiency and the molecular weight, relative quantity, and other properties of the transferred proteins. This section provides an overview of total protein stains, including anionic dyes (such as amido black, Coomassie blue, Ponceau S, and Fast Green), fluorescent stains, and colloidal gold stains and discusses the advantages and disadvantages of these stains. It also provides protocols for total protein staining and troubleshooting tips for various problems in staining procedures.
Related Topics: Immunodetection.
Page Contents
When performing total protein blot staining, note that:
- Protein standards are useful for monitoring transfer efficiency and serve as molecular weight markers for calibration of blot patterns. Refer to protein standards section for available Bio-Rad protein standards.
- Polyacrylamide gels shrink during staining, so comparison of an immunologically probed membrane to a stained gel is not practical. To determine the exact location of a specific antigen in relation to other proteins, compare two blotted membranes, one that has been probed with an antibody and the other stained for total protein.
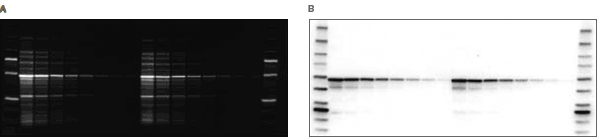
Total protein detection. Blot stained with SYPRO Ruby blot stain showing the total protein pattern of an E.coli lysate containing an over-expressed GST fusion protein on the blot.
Comparison of total protein staining methods.
| Method | Sensitivity | Advantages | Disadvantages | Imaging |
| Anionic dyes (Ponceau S, Coomassie Brilliant Blue R-250, amido black, Fast Green FCF) | 100–1,000 ng | Inexpensive, rapid | Low sensitivity, shrink membrane | Photography with epi-illumination or reflectance densitometry |
| Fluorescence | 2–8 ng | Sensitive, mass spectrometry-compatible | Fluorescence detection system required | Fluorescence visualization with UV, LED epi-illumination, or laser scanning |
| Stain-free (SFX) | 2–28 ng | Rapid – no additional staining or destaining required | Special gels and imaging equipment required | Gel Doc™ EZ system |
| Colloidal gold (enhanced) | 100 pg–1 ng | Very sensitive, rapid; optional enhancement increases sensitivity | Expensive | Photography with epi-illumination or reflectance densitometry |
The first techniques developed for total protein staining of blotted membranes used the same anionic dyes commonly used for staining proteins in polyacrylamide gels. These dyes include amido black (Towbin et al. 1979), Coomassie (Brilliant) Blue R-250 (Burnette 1981), Ponceau S, and Fast Green FCF (Reinheart and Malamud 1982). Of these:
Amido Black — destains rapidly in acetic acid/isopropanol solution and produces very little background staining. Amido black may interfere with downstream immunodetection.
Coomassie Brilliant Blue — may show high background staining, even after long destaining procedures, and is not compatible with subsequent immunodetection.
Ponceau S and Fast Green — are compatible with downstream immunodetection methods, and Fast Green can be easily removed after visualization to allow subsequent immunological probing.
These dyes are easy to prepare and they stain proteins quickly, but they are relatively insensitive when compared to other stains (see table above).
Fluorescent stains such as SYPRO Ruby and Deep Purple provide highly sensitive detection of proteins on blots as well as in gels. SYPRO Ruby blot stain allows detection as low as 2 ng. After staining, target proteins can be detected by colorimetric or chemiluminescence immunodetection methods, or analyzed by microsequencing or mass spectrometry with no interference from the protein stain.
Stain-free technology enables the visualization of proteins in gels and on membranes without a staining step and provides several advantages over conventional staining procedures. In particular, it eliminates the need for a lengthy destaining step, which makes visualization faster and more efficient and eliminates the need to dispose of organic waste generated during destaining.
Stain-free technology also offers tremendous advantages in quantitative western blotting experiments. Using a stain-free enabled imager, total protein normalization can be performed on posttransfer membranes, allowing for precise quantitation of western blotting signals. Total protein normalization is becoming the gold standard for western blotting normalization in major peer-reviewed journals.
Learn more about the advantages of using stain-free technology.
Colloidal gold is an alternative to anionic dyes that provides detection sensitivities rivaling those of immunological detection methods (Moeremans et al. 1987, Rohringer and Holden 1985). When a solution of colloidal gold particles is incubated with proteins bound to a nitrocellulose or PVDF membrane, the gold binds to the proteins through electrostatic adsorption. The resulting gold-protein complex produces a transient, reddish-pink color due to the optical properties of colloidal gold. This gold-protein interaction is the basis for total protein staining with colloidal gold as well as for specific, immunogold detection (see Immunogold Labeling)
Comparison of total protein staining methods.
| Problem | Cause | Solution |
| Colloidal gold total protein stain — high background | The blocking step was insufficient or was omitted |
|
| Contamination occurred during electrophoresis or transfer |
|
|
| Excessive amounts of protein were loaded on the gel or too much SDS was used in the transfer buffer. Proteins can pass through the membrane without binding and recirculate through a tank blotting system |
|
|
| The colloidal gold stain solution was contaminated |
|
|
| The development step was too long |
|
|
| Colloidal gold total protein stain — low sensitivity | The incubation time was insufficient |
|
| Transfer was incomplete |
|
|
| The stain was exhausted, as evidenced by the loss of the dark burgundy color and longer staining times |
|
|
| Buffer salt contamination has occurred; the solution is light blue instead of dark burgundy |
|
|
| Anionic dyes — high background | Destaining was insufficient |
|
| The dye solution was too concentrated |
|
|
| Anionic dyes — low sensitivity | Anionic dye stains do not detect protein bands below ~100 ng |
|
| Fluorescent blot stains — low sensitivity | Proteins with low hydrophobicity |
|
| Incorrect excitation and emission settings were used |
|
Burnette WN (1981). "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112, 195–203.
Moeremans M et al. (1987). The use of colloidal metal particles in protein blotting. Electrophoresis 8, 403–409.
Reinhart MP and Malamud D (1982). Protein transfer from isoelectric focusing gels: the native blot. Anal Biochem 123, 229–235.
Rohringer R and Holden DW (1985). Protein blotting: detection of proteins with colloidal gold, and of glycoproteins and lectins with biotin-conjugated and enzyme probes. Anal Biochem 144, 118–127.
Towbin H et al. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76, 4350–4354.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6216 | Detection Buffer Formulations | Click to download |
| 6217 | Total Protein Detection | Click to download |
| 6218 | Blot Stripping & Reprobing | Click to download |

