On This Page |
Characterize with Confidence | Viral Vector Characterization | Transgene and Cell Characterization | Recombinant Anti-Idiotypic Antibodies | Resources |
Maximize Quantitative Insight
Develop critical assays to evaluate potency and batch-to-batch consistency, whether for CAR T-cell characterization or development of another cell therapy approach.
Blend Efficiency with Quantitative Accuracy in Your Cell Therapy Characterization Assays
While a number of established therapeutic approaches start with biological materials, and all their inherent variability, cell therapies bring the complexity to the next level. From the necessarily personalized approach for autologous therapies and their small-scale production to the complexities of transgene constructs that need to be optimized across numerous parameters, cells are an exceedingly challenging material to turn into a standardized therapeutic.
The answer to this challenge is, of course, to deeply characterize each cell therapy batch. With reliable, quantitative measurement of numerous cellular parameters you can reach for the standardized ideal.
As you look for the most efficient ways to develop reliable and quantitative characterization assays, you can turn to our proven solutions. With products and services that combine ease-of-use and efficiency with high-performance, we can help you maximize your quantitative insight for reliable CAR T-cell characterization and more.

Did you know?
Emily Whitehead, the first pediatric patient to be treated with a CAR T-cell therapy, remains cancer-free ten years after treatment.1 Her parents brought her to Children’s Hospital of Philadelphia to treat rapidly progressing acute lymphoblastic leukemia (ALL) and she was the first patient to enroll in Phase 1 clinical trials, led by Stephan Grupp, MD. PhD., for the CAR T-cell therapy.
Characterize Your Transgene Delivery Vector
Assess Viral Purity
To assess viral vector purity at later stages of development where sensitivity and quantitative accuracy are needed to ensure safety and quality, Droplet Digital™ PCR (ddPCR™) assays can deliver the confidence of absolute quantification for detecting residual host cell DNA or pathogenic microbes.
Discover Solutions for Reliable and Accurate Impurity Testing »
Measure Vector Copy Number to Assess Infectivity
In contrast to viral titer measurement with qPCR, quantification with ddPCR technology does not depend on amplification efficiency but rather is an endpoint PCR assay that delivers absolute quantitation. As a result, measurement with ddPCR technology is more precise and accurate than qPCR and with Bio-Rad’s family of ddPCR instruments, vector copy number determination using ddPCR technology is straightforward to learn and implement.
Learn about Vector Copy Number Measurement »
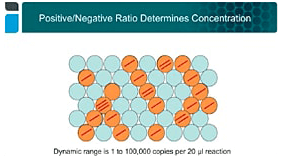
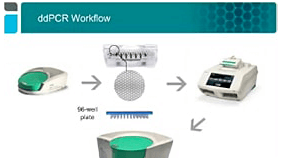
Introduction to Droplet Digital PCR: Workflow and Applications
Explore the concept, workflow, and performance of Droplet Digital PCR and diverse applications showcased in recent journal publications.
Characterize Your Cell
From early discovery investigations to developing critical quality attribute assays, we offer time-saving tools for assessing protein expression, characterizing the surface of your cells, and quantifying cell viability.
For early transgene expression assessment, our Stain-Free Western Blotting technology combines speed with quantitation.
Learn about Using Stain-Free Technology to Quantify Transgene Expression »
For high-throughput, multi-parameter analysis of protein expression in specific cell subpopulations, our ZE5 Cell Analyzer puts high-performance into an automation-ready instrument. You can quickly obtain the deep level of information you need to assess discrete immune cell populations.
- Distinguish T cells during isolation and activation
- Evaluate CAR expression on functional T cells
- Monitor CAR T-cell potency and specificity
Learn More about the ZE5 Cell Analyzer »
Learn how flow cytometry can be used for CAR T-cell therapy development »
Featured Publication CAR T-Cell Characterization
Utilizing CRISPR-mediated genetically modified CAR T cells to treat T-cell malignancies without CAR T-cell fratricide.
Detect with the Unlimited Confidence of Recombinant, Anti-Idiotypic Antibodies
When the assays you develop are being used to make critical decisions on which CAR construct to advance or if the potency of a cell therapy batch meets the standards you have set for quality, you need to be confident in their robustness and reproducibility.
With custom recombinant monoclonal anti-idiotypic antibody generation from Bio-Rad, you get fast development of highly specific antibodies that you can rely on throughout the lifecycle of your assays. You also get expert support from a scientific team that has been making recombinant anti-idiotypic antibodies and advancing recombinant antibody production technology for over a decade.
Bio-Rad's custom recombinant, anti-idiotypic antibody service offers:
- Antibody specificity against the unique determinants of the CAR
- Multiple format and conjugation options from the outset using SpyTag technology
- Antibodies dispatched in less than 3 months
- Recombinant production — reliable, consistent quality, easy scale-up
- Antibody sequence available for security and independence
Learn about Our Custom Anti-Idiotypic Antibody Generation Service »
Resources
Webinars
-
Introduction to Droplet Digital™ PCR: Workflow and Applications
Learn the concept, workflow, and diverse applications of ddPCR technology showcased in recent journal publications.
-
Droplet Digital™ PCR Tips and Tricks: ddPCR Assay Design
Find tips and tricks used in ddPCR assay design. Topics covered include the availability of commercial ddPCR assays.
Publications
Insights on Droplet Digital PCR-Based Cellular Kinetics and Biodistribution Assay Support for CAR-T Cell Therapy
Sugimoto H, et al. AAPS J. 2021 Mar 2;23(2):36. doi: 10.1208/s12248-021-00560-6.
Application of Droplet Digital PCR for the Detection of Vector Copy Number in Clinical CAR/TCR T Cell Products
Lu A, et al. J Transl Med. 2020; 18: 191.
Documents
-
Droplet Digital™ PCR (ddPCR™) Is Well Suited for Quantifying Transgene Copy Number
Discover how ddPCR technology is the ideal solution for transgene quantification and increases CAR T-cell development success.
-
Automation of High-Throughput Flow Cytometry with the ZE5 Cell Analyzer
Learn how integrating flow cytometers into automated workcells increases throughput, enables efficient operation, and provides consistent results.
-
Counting Genetically Modified Cells Using Whole Cell-Based Droplet Digital™ PCR
Count modified cells using ddPCR technology by encapsulating whole cells and targeting the transgene and reference gene to amplify DNA within.
References
- Children’s Hospital of Philadelphia. Emily Whitehead, First Pediatric Patient to Receive CAR T-Cell Therapy, Celebrates Cure 10 Years Later. Published May 10, 2022. Accessed July 20, 2022. https://www.chop.edu/news/emily-whitehead-first-pediatric-patient-receive-car-t-cell-therapy-celebrates-cure-10-years
Discover More Cell Therapy Development Solutions
-
Cell Therapy Development Overview
Creating standardized treatments from inherently variable material like living cells requires knowing exactly what you are working with, whether in discovery, development, or manufacturing. Bio-Rad is here to help.
-
Antigen Discovery
Discover innovative cell therapy approaches quickly and confidently with automated and quantitative tools for neoantigen discovery, CAR T-cell characterization, CAR construct optimization, and more.
-
In Vivo Bioanalysis
Assess the body’s reaction to your cell therapy by developing in vivo bioanalysis assays to monitor CAR T-cell persistence, exhaustion, and other functional parameters.
-
Manufacturing & QC
Efficiently ensure the safety of your cell therapy manufacturing process, whether you are evaluating transgene copy number or verifying the absence of microbial pathogens.