Protein solubilization can be achieved by the use of chaotropic agents, detergents, reducing agents, buffers, and/or ampholytes. The various components of sample buffers, such as chaotropic agent, detergents, carrier ampholytes and reducing agents are discussed in the following. Find protocols for sample solubilization and preparation in the Documents and Protocols section below.
Related Topics: Protein Extraction and Cleanup and Protein Fractionation and Depletion.
Page Contents
The table below lists the relevant products from Bio-Rad that can be used in sample preparation for 2-D electrophoresis.
| Sample Preparation Products, Solutions or Kits | Chaotropic Agent | Detergent | Reducing Agent | Buffer | Ampholyte | |
| 163-2100 | ReadyPrep™ Sequential Extraction Kit | • | • | • | • | • |
| 163-2102 | ReadyPrep Reagent 1, 1 vial | — | — | — | • | — |
| 163-2103 | ReadyPrep Reagent 2, 1 vial | • | • | • | • | • |
| 163-2104 | ReadyPrep Reagent 3, 1 vial | • | • | • | • | • |
| 163-2105 | ReadyPrep 2-D Starter Kit | • | • | • | • | • |
| 163-2106 | ReadyPrep 2-D Starter Kit Rehydration/Sample Buffer | • | • | • | • | • |
| 163-2141 | Microrotofor Cell Lysis Kit (Mammal) | • | • | • | • | • |
| 163-2142 | Microrotofor Cell Lysis Kit (Plant) | • | • | • | • | • |
| 163-2143 | Microrotofor Cell Lysis Kit (Yeast) | • | • | • | • | • |
| 163-2144 | Microrotofor Cell Lysis Kit (Bacteria) | • | • | • | • | • |
| Individual Components | ||||||
| 161-0730 | Urea, 1 kg | • | — | — | — | — |
| Thiourea | • | — | — | — | — | |
| 161-0460 | CHAPS, 1 g | — | • | — | — | — |
| 161-0465 | CHAPSO, 1 g | — | • | — | — | — |
| SB 3-10 | — | • | — | — | — | |
| 161-0407 | Triton X-100, 500 ml | — | • | — | — | — |
| 161-0611 | 1 Dithiothreitol, 5 g | — | — | • | — | — |
| 163-2101 | 1 Tributylphosphine, 200 mM, 0.6 ml | — | — | • | — | — |
| 161-0716 | Tris, 500 g | — | — | — | • | |
| 163-1112 | Bio-Lyte® 3/10 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1132 | Bio-Lyte 3/5 Ampholyte, 20%, 10 ml | — | — | — | — | • |
| 163-1142 | Bio-Lyte 4/6 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1152 | Bio-Lyte 5/7 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1192 | Bio-Lyte 5/8 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1162 | Bio-Lyte 6/8 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1172 | Bio-Lyte 7/9 Ampholyte, 40%, 10 ml | — | — | — | — | • |
| 163-1182 | Bio-Lyte 8/10 Ampholyte, 20%, 10 ml | — | — | — | — | • |
| Bio-Lyte IEF Buffers* | ||||||
| 163-2093 | 100x ReadyStrip 7–10 Buffer, 1 ml | — | — | — | — | • |
| 163-2094 | 100x Bio-Lyte 3/10 Ampholyte, 1 ml | — | — | — | — | • |
| 163-2095 | 100x ReadyStrip 6.3–8.3 Buffer, 1 ml | — | — | — | — | • |
| 163-2096 | 100x ReadyStrip™ 5.5–6.7 Buffer, 1 ml | — | — | — | — | • |
| 163-2097 | 100x ReadyStrip 4.7–5.9 Buffer, 1 ml | — | — | — | — | • |
| 163-2098 | 100x ReadyStrip 3.9–5.1 Buffer, 1 ml | — | — | — | — | • |
* Dilute ReadyStrip buffers to 1x final in each sample to equal 0.2% Bio-Lyte ampholyte.
Urea is the most commonly used chaotropic agent in sample preparation for 2-D PAGE. Thiourea can be used to help solubilize many otherwise insoluble proteins. Urea and thiourea disrupt hydrogen bonds and are used when hydrogen bonding causes unwanted aggregation of formation of secondary structures that affect protein mobility. Urea is typically used at 8M. Thiourea is weakly soluble in water, but is more soluble in concentrated solutions of urea, so a mixture of 2 M thoiurea and 5–8 M urea is used when strongly chaotropic conditions are required (Rabilloud 1998).
Detergents are added to disrupt hydrophobic interactions and increase solubility of proteins at their pI. Detergents must be nonionic or zwitterionic to allow proteins to migrate according to their own charges. Some proteins, especially membrane proteins, require detergents for solubilization during isolation and to maintain solubility during focusing. Ionic detergents such as SDS are not compatible with IEF, but can be used with concentrated samples in situations where the SDS can be unbound from the protein by IEF-compatible detergents that compete for binding sites. Nonionic detergents such as NP-40 and Triton X-100 are not very effective in solubilizing hydrophobic proteins; zwitterionic detergents such as CHAPS and sulfobetaines (for example, SB 3–10 or ASB 14) provide higher solubilization efficiency, especially for integral membrane proteins.
A fundamental challenge with IEF is that some proteins tend to precipitate at their pI. Even in the presence of detergents, certain samples may have stringent salt requirements to maintain the solubility of some proteins. Salt should be present in a sample only if it is an absolute requirement, and then only at a total concentration less than 40 mM. This is problematic since any salt included will be removed during the initial high-current stage of focusing. Salt limits the voltage that can be applied without producing high current, increasing the time required for focusing. Proteins that require salt for solubility may precipitate once the salt is removed. Carrier ampholytes sometimes help to counteract insufficient salt in a sample. They are usually included at a concentration of < 0.2% (w/v) in sample solutions for IPG strips. High concentrations of carrier ampholytes will slow down IEF until they are focused at their pI, since they carry current and hence limit voltage.
Some researchers have increased resolution by varying the ampholyte composition. An example is shown in the figure below, where the resolution in the first dimension is greatly increased by using a mixture of ampholytes. See table for relevant products from Bio-Rad.
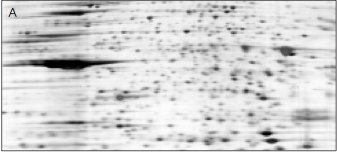
carrier ampholytes, pH 3–10, 80,000 V-hr

carrier ampholytes, pH 5–8/8–10 (2:1), 80,000 V-hr
Effect of ampholytes on resolution. Matching sections of 2-D images are shown. In both A and B, 110 μg of a cytosolic extract of a human lymphoblastoid cell line was passively loaded into a 17 cm pH 5–8 ReadyStrip IPG strip. Second-dimension separation was in 10–24% gradient gels with PDA crosslinker in PROTEAN® II XL format. In A, pH 3–10 carrier ampholytes were used. In B, pH 5–8 carrier ampholytes were mixed with pH 8–10 carrier ampholytes at a 2:1 ratio. The use of the ampholyte mixture greatly improved focusing. Data kindly provided by R Joubert-Caron, Laboratoire de Biochimie des Proteines et Proteomique.
Reducing agents such as dithiothreitol (DTT) or tributylphosphine (TBP) are used to disrupt disulfide bonds. Bond disruption is important for analyzing proteins as single subunits. DTT is a thiol reducing agent added in excess to force equilibrium toward reduced cysteines. At 50 mM it is effective in reducing most cysteines, but some proteins are not completely reduced by this treatment. If the concentration of DTT is too high it can affect the pH gradient since its pKa is around 8. The figure below shows the effect of DTT concentration on samples of soluble E.coli proteins. The result will be different for samples from different sources.
TBP is a much more effective reducing agent than DTT. It reacts to reduce cysteines stoichiometrically at low millimolar concentrations (Herbert et al. 1998). It is chemically more difficult to handle than DTT. See product table for these reducing agents from Bio-Rad.
For a more thorough discussion of the effects of detergents, denaturing agents, and reducing agents on protein solubility, consult the following papers: Rabilloud (1998, 1999), Herbert et al. (1998), Molloy (2000), and Taylor et al. (2000).

Effect of DTT concentration on 2-D protein spot pattern. A, 200 μg of E. coli extract was suspended in rehydration buffer containing 10 mM DTT and subjected to 2-D gel electrophoresis (first dimension in 11 cm, pH 4–7 IPG for 40,000 V-hr, second dimension in 8–16% SDS-PAGE gel). In B, C, and D, rehydration buffer included 25 mM, 50 mM, and 100 mM DTT, respectively. The gels were stained with Bio-Safe™ Coomassie Blue stain. The images show the acidic, low MW regions of each gel. Notice that as the DTT concentration was increased, the number of spots resolved in this region also increased, indicating that 10 mM DTT is insufficient to completely reduce the disulfides present in the protein mixture. Data kindly provided by William Strong of Bio-Rad Laboratories.
Explore the most commonly used methods for protein solubilization for 1-D SDS-PAGE applications.
Learn more about the different assays used for protein quantitation on SDS-PAGE gels.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6200 | Sample Quantitation (RC DC Protein Assay) Protocol and Compatibility Table | Click to download |
| 6221 | Sample Quantitation (RC DC Protein Assay) | Click to download |
| 6220 | Solubilization | Click to download |
