Process Separations Webinars

On This Page |
Ask an Expert — 15 Minutes | Past Webinars |
Ask an Expert — 15 Minutes
Evaluation of a New Multimodal Anion Exchange Resin for Emerging Biotherapeutic Process Challenges
This webinar discusses biomolecules that were purified using Nuvia wPrime 2A Media with focus placed on proteins with low pI and known strong binding to alternative mixed-mode anion exchange resins.
-
Exploring the Use of Multimodal Chromatography for the Purification of mRNA Vaccines
mRNA vaccines offer high-yield, cell-free production but present significant challenges in impurity removal, particularly dsRNA, which can trigger strong immune responses and inhibit translation. This webcast explores the use of multimodal chromatography to selectively separate dsRNA and other impurities from mRNA, achieving >80% recovery and >88% purity in a single step.
-
Two-Step mAb Purification Using an AEX-HIC Mixed-Mode Resin
Learn how this innovative approach simplifies traditional three-step purification by eliminating an entire chromatography step—saving time, buffers, and media costs while enhancing efficiency.
-
Demonstrating a Modular Selectivity With a Weak AEX-HIC Resin for Achieving High mAb Purity and Recovery
Learn how Bio-Rad's new mixed-mode media, Nuvia wPrime 2A, with a modulable selectivity was employed for designing a mAb purification process in a flow-through mode.
-
Effective Strategies for Endotoxin Removal in Therapeutic Protein Purification
Endotoxins complicate therapeutic protein purification and can cause severe reactions. They hinder research data accuracy and are hard to remove due to thermal stability and pH insensitivity. This webcast covers successful case studies for endotoxin removal
-
Enhancing Process Development with Viral Clearance: mAb Purification With a Weak AEX-HIC Chromatography Resin
A viral clearance study was performed to showcase the capabilities of a new weak AEX-HIC resin and in all tested conditions, no residual infectivity was detected in the end products.
-
Strategies to Address Large Molecule Purification Challenges
This webinar delves into innovative strategies to surmount these challenges, employing design of experiments (DOE) methodologies and offering insightful case studies centered around plasma protein and virus purification. These real-world applications showcase tangible enhancements in productivity and overall process economics within large molecule purification.
-
CHT Chromatography Media: 5 Essential Facts
For over 30 years, CHT™ Ceramic Hydroxyapatite Chromatography Media has been a trusted, proven purification solution for many biopharmaceutical manufacturing processes. CHT provides exceptional removal of impurities when other chromatography resins fall short to provide the same level of purity.
-
AAV Purification Using AEX and Mixed-Mode Chromatography
This webcast presents a study exploring different chromatography workflow solutions for purifying recombinant adeno-associated viruses (rAAV), a promising vector for gene therapy due to its safety and potential applications.
-
mAb Purification Process Development Using Mixed-Mode Resins
This webcast covers DOE strategies for process development, including screening, modeling, and optimization, and the selection of models and tools for each stage, as well as data evaluation using mixed-mode resins.
-
A Polishing Strategy for Removing Impurities in Bispecific Antibody Purification
A study using asymmetric and symmetric IgG-like bsAb constructs demonstrated an effective polishing strategy using CHT™ Media. The method achieved excellent removal of byproducts and impurities.
-
Development of IEX Purification Process for Lentiviral Vectors
This webcast explores the development of LVV using chromatographic adsorbents, utilizing high-throughput screening to anion exchange resins.
-
Purification of DNA Oligonucleotides using Anion Exchange Chromatography
This webcast presents data on the purification of DNA oligonucleotides using Nuvia HP-Q Resin, a high-performance strong anion exchanger.
-
Purification of Biosimilar Insulin from E. coli
This webcast presents new purification workflow for recombinant human insulin from E. coli using CEX resins.
-
CHT Prepacked Process-Scale Columns
This webcast showcases performance data for GMP ready CHT prepacked process-scale columns, demonstrating excellent column performance and shipping stability
-
Alternative to a Three-Step mAb Purification Process
This webcast compares a three-step mAb purification to a two-step mAb purification workflow using mixed-mode chromatograpy.
-
What You Need to Know About Packing Resins at Process Scale
Dr. Mark A. Snyder, to learn best practices and guidelines for successfully packing different types of chromatography resins.
-
Manufacturing a Recombinant Retrovirus — Downstream Purification Process Development
This webcast details the importance of resin screening, endonuclease treatment, process development, and the scale-up purification of a retrovirus-like particle intended for use in human subjects.
-
Mixed-Mode Chromatography — Optimizing Target Purity and Recovery with Buffer Additives
This webcast showcases the unique selectivity of mixed-mode chromatography and detail the use of buffer additives for optimal target purity and yield.
-
Efficient and Rapid Purification of E. coli Expressed Toxin Recombinant Protein Fragments
Dr. Lees presents the purification process for rTTHc using polymeric chromatography resins. rTTHc was expressed at high levels as a soluble, properly folded intracellular protein in Fina Bio proprietary Escherichia coli strain.
-
Assessing Viral Clearance in Early Phase Process Development
Viral clearance studies are part of a multifaceted approach to ensure the safety of biopharmaceutical products. In order to prevent costly changes to a manufacturing process, it is important to assess each operation unit for its efficiency on the removal or inactivation of adventitious agents early on during downstream process development.
-
Mixed-Mode Chromatography Resins for Biomolecule Purification
This webinar examines mixed-mode chromatography for biomolecule purification, which has emerged as a viable purification method for biomolecules that are otherwise difficult to purify using traditional platforms and other established means.
-
Virus Purification Strategies and Considerations
Alternative strategies are needed for achieving efficient virus purification. This webinar will provide a snapshot of the resins considered, and the reasons for their selection, in order to achieve the required yield and purity.
-
AEX Mixed-Mode Chromatography Resin for High Selectivity and Recovery
This webinar introduces the features of Nuvia aPrime 4A Resin, for high-purity and recovery over traditional chromatography resins.
-
CHT Column Packing is Easier Than You Think
This webinar presents best practices and guidelines for packing CHT Ceramic Hydroxyapatite Media at a process scale.
-
Nuvia HP-Q — A New High Performance AEX Resin
In this webinar, Dr. Jiali Liao presents case studies and design features to meet manufacturing demands.
Past Webinars
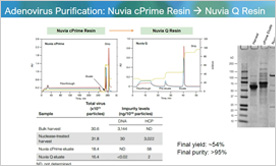
A Mixed-Mode Approach — Alternative to a Three-Step mAb Purification Process
In this webinar, we evaluate three monoclonal antibody purification workflows, compare alternatives to the traditional mAb three-step process focused on time and cost reduction, and we explore functionalities of mixed-mode resins.
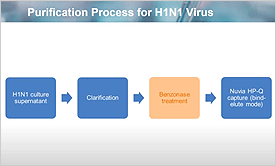
Single-Step Column Purification for H1N1 Virus Using IEX Chromatography Resin
We discuss the development of vaccine production process against the Influenza A H1N1 subtype, using recently developed ion exchange chromatography resins.
Two-Step Purification of Low-Expressing Recombinant Exoprotein A Using Mixed-Mode Chromatography
This case study demonstrates an efficient purification workflow for a low-expressing recombinant protein.

Purification Strategies for Vaccine Production and Gene Therapy
Dr. Mark Snyder discusses strategies when developing purification methods for vaccine production and gene therapy.

A New Mixed-Mode Media for Biomolecule Purification
Drs. Yoshikawa and Snyder discuss application uses and provide selection guidelines to start your purification.
Integrating Viral Clearance into Your Process Development — A Case Study Using a Mixed-Mode Chromatography Resin
Dr. Iheanacho and Dr. He present a case study using a DOE approach with a mixed-mode chromatography resin to evaluate viral clearance.
Large Biomolecule Purification with a New High-Performance Anion Exchange Resin
Drs. Kim and Liao introduce the design features of the Nuvia HP-Q Bead Matrix that help overcome purification challenges.