Our Best-in-Class
COVID-19 Solutions
Reliable Detection and Differentiation
-
COVID-19 Serology Testing for IVD
Platelia SARS-CoV-2 Total Ab Assay
As COVID-19 continues to impact countries worldwide, accurate screening is needed to establish serological status of different populations. Bio-Rad now offers the Platelia SARS-CoV-2 Total Ab assay, for the detection of total anti-nucleocapsid antibodies (IgM, IgA, IgG) to SARS-CoV-2. In one test, the assay helps identify the immune response to coronavirus SARS-CoV-2, the virus associated with COVID-19.
The following includes product offerings in certain areas. Some products have limited regional availability. If you have a specific question about our COVID-19 solutions, please contact your local sales office or representative.
Contact a Specialist -
Our Best-in-Class COVID-19 Solutions
- Real-Time PCR instruments and reagents
- SARS-CoV-2 standards
- ddPCR instruments and reagents
- SARS-CoV-2 ddPCR kit (CE-IVD)
-
Aids in Diagnosis and Patient Surveillance
The Platelia SARS-CoV-2 Total Ab assay detects total antibodies against SARS-CoV-2, the virus that causes COVID-19, to help aid in diagnosing new infections as well as provide a more complete understanding of infection rates.

On Demand Webinar
Understanding the Role of Serology Assays in COVID-19 Diagnostics and Surveillance
Jean-Fancois Mouscadet
Director of Research and Development
This webinar focuses on the role of serology tests in the pandemic response as well as the clinical value of a Total Antibody assay
View on DemandPlatelia SARS-CoV-2 Total Ab Assay — At a Glance
Compliant with regulatory standards (CE-IVD validated)
COVID-19
Platelia SARS-CoV-2 Total Ab Assay:
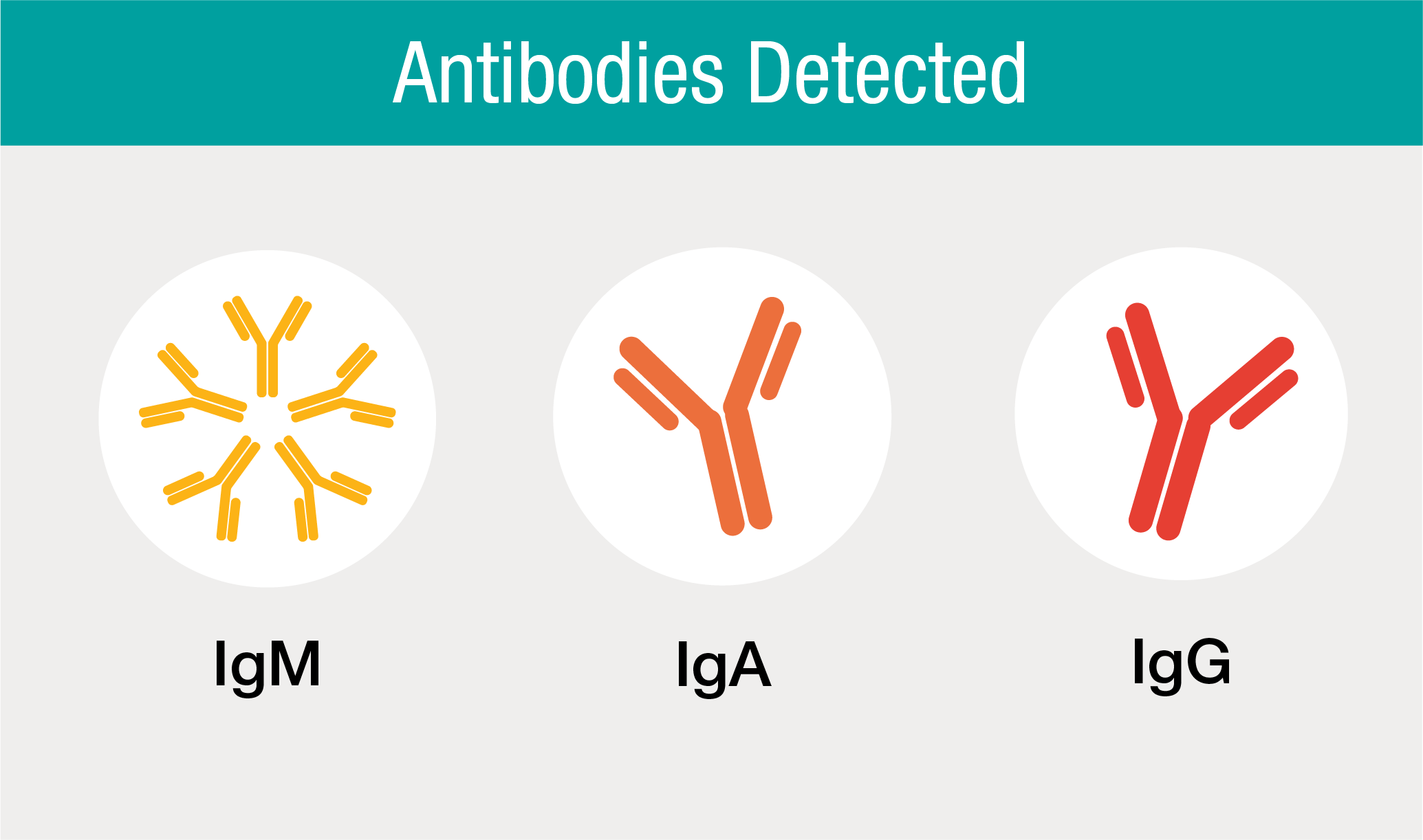
Detection of IgM, IgA and IgG in One Test
The Platelia SARS-CoV-2 Total Ab assay is a high performance screening immunoassay for the detection of anti-nucleocapsid IgM, IgA and IgG antibodies to SARS-CoV-2.
Convenient and Fast
- 1-step antigen capture format (only 90-min incubation time required)
- 96-well microplate testing with breakable strips
- Ready to use with colored reagents
- Visual control of sample dilution and reagent addition
Total Anti-Nucleocapsid Antibody Detection for SARS-CoV-2
The Total Antibody immunoassay format is based on the detection of total antibodies – IgM, IgA and IgG – against the nucleocapsid protein (N-protein) of SARS-CoV-2, all in just one test. The antibodies IgM and IgA are detectable in the case of acute SARS-CoV-2 infection while IgG is detectable in the recovery phase or post infection. Combining the results from these phases delivers a Total Antibody positive result.

Disclaimer: This serological profile is based on projections from other well-known infections and may not fully reflect the course of SARS-CoV-2 infection.

Total Ab Detection vs. Single Ab Detection
Studies by Zhao et al., 2020 demonstrated that total antibody detection with antibodies like IgM, IgA and IgG is associated with better sensitivity, regardless of the time after symptom onset, by comparison to IgM or IgG detection only.

Why Target the Nucleocapsid Protein?
The Nucleocapsid protein (N-protein) is the most abundant SARS-CoV-2 protein and is a significant marker for diagnostic testing assays. Studies in 2020 show that anti-nucleocapsid antibodies (IgM and IgG) can be produced at higher levels and detected very early in the acute infection in comparison to anti-spike antibodies.3,4 ELISA detection of anti-nucleocapsid antibodies is associated with higher sensitivity by comparison to anti-spike antibody detection.1,2

Accurate Performance
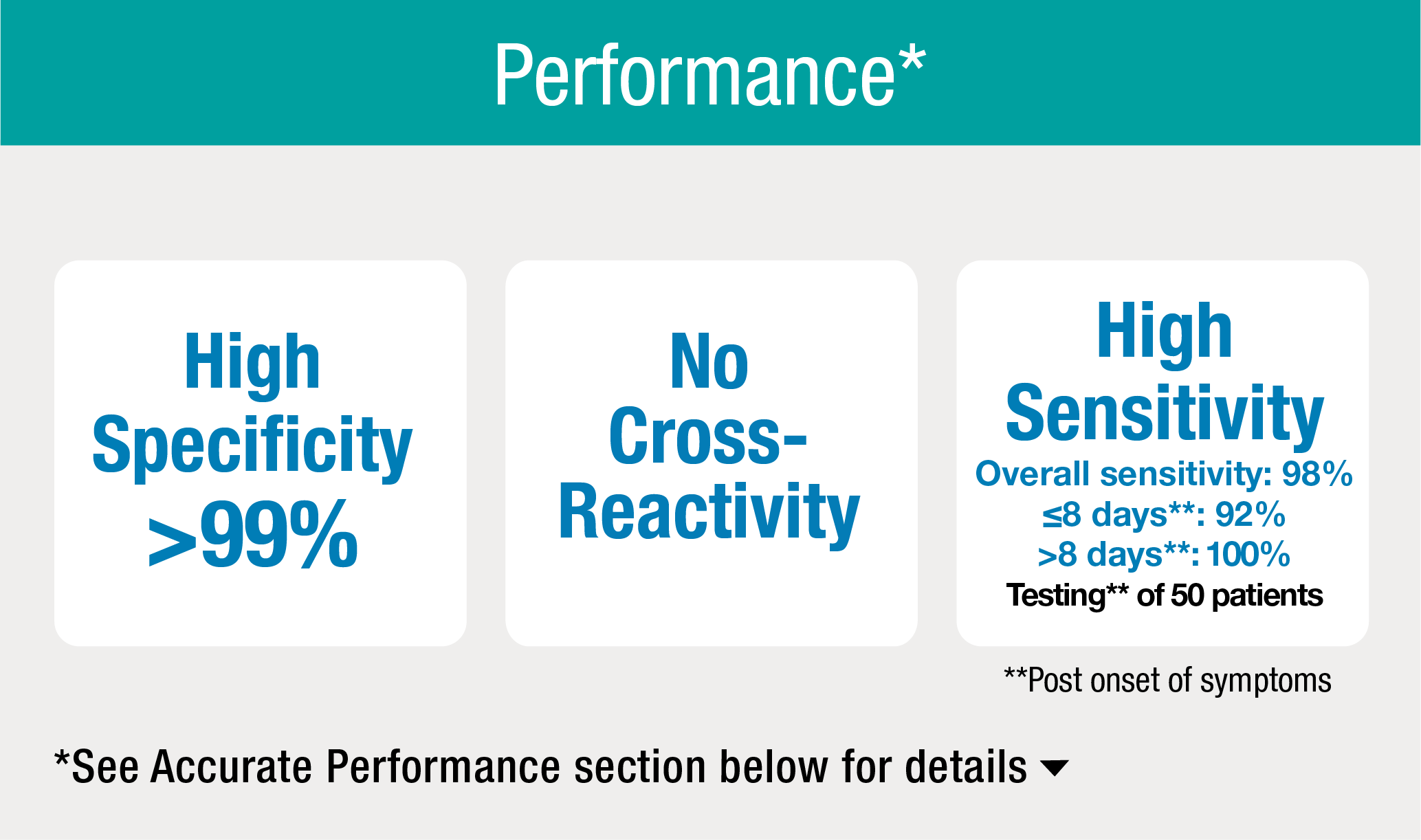
High Specificity
The assay showed >99% specificity for specimens from 500 blood donors and 100 hospitalized patients negative for SARS-CoV-2. A total of 600 specimens were tested.
When negative specimens are tested with the Platelia SARS-CoV-2 Total Ab assay, strongly negative values are obtained. The high discrimination between negative and positive values is associated with accuracy and reliability of the results.

No Cross-Reactivity
The assay showed no cross-reactivity to four of the most common coronaviruses, along with EBV, CMV, RSV, flu vaccine and upwards of 25 other medical conditions. A total of 168 specimens were tested.
High Sensitivity
92% sensitivity was achieved for patients tested ≤ 8 days after onset of symptoms and 100% sensitivity for patients tested > 8 days after onset of symptoms. A total of 50 patients were tested.
Thanks to Total Antibody detection, the Platelia SARS-CoV-2 Total Ab assay is associated with earlier detection of seroconversion and consistent detection of the patient’s adaptive immune response over time. With just one test, the Platelia SARS-CoV-2 Total Ab assay reliably detects patients that have been exposed to SARS-CoV-2.

-
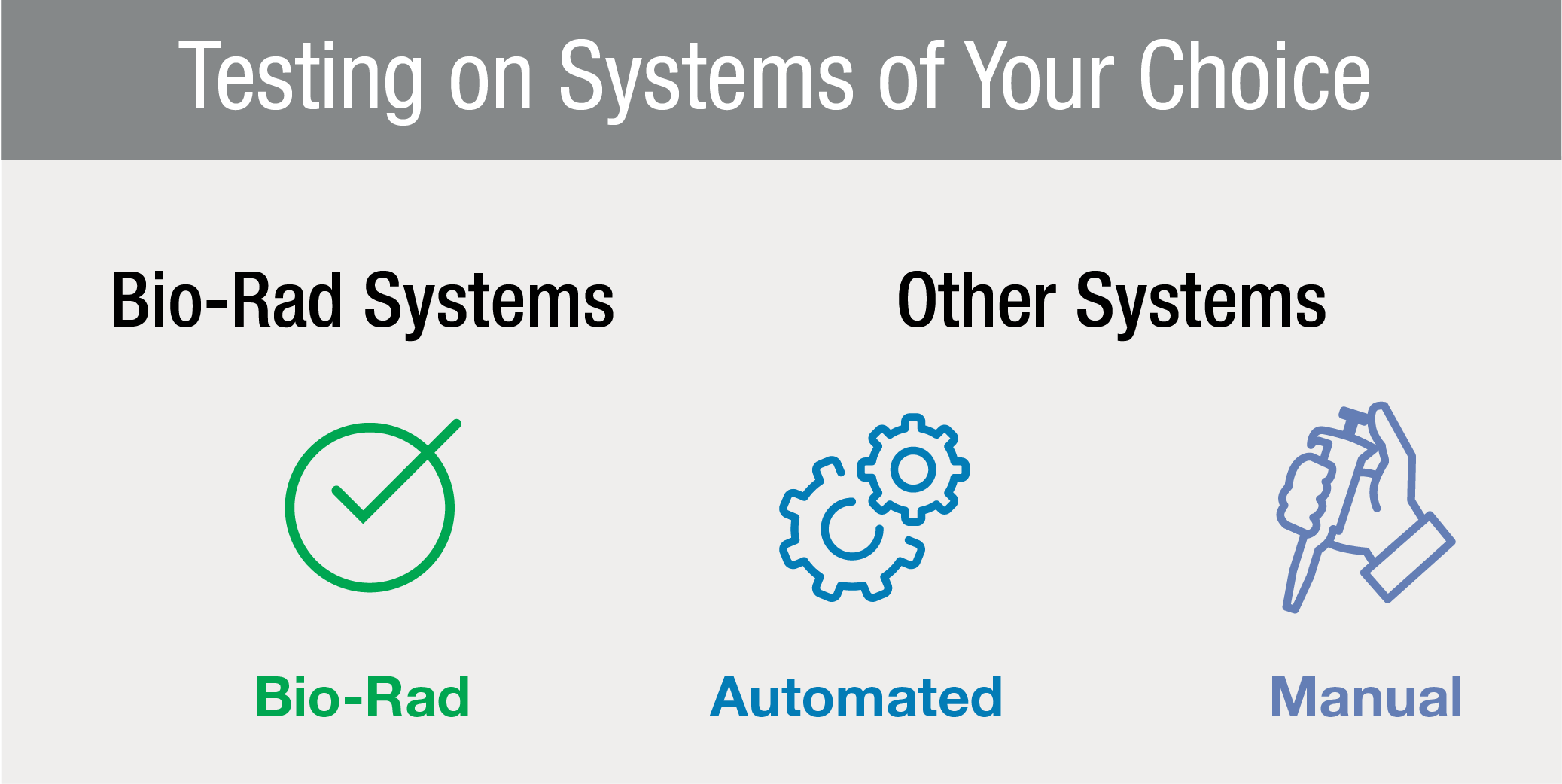
Testing on Systems
of Your ChoiceThe Platelia SARS-CoV-2 Total Ab assay is designed for convenient processing on your choice of automated microplate processors. While the assay is recommended for use on the Bio-Rad validated, fully-automated EVOLIS Systems or stand-alone systems (IPS/PR4100/PW40), it can also be run on other validated manual or automated platforms.
-
Bio-Rad EVOLIS Advantages
Validated for Platelia SARS-CoV-2 Total Ab assay testing, Bio-Rad instruments feature automated, scalable processing, full results traceability, and high safety standards.
Download Brochure 
User-friendly
Intuitive software automates each assay step to reduce operator intervention
Short Time-to-Result
Results obtained in 2 hr 30 min
Optimal Workflow
- Screening of 360 patients in an 8-hour work shift
- Ability to directly load kit reagents
- Possibility to scale up productivity by combining multiple EVOLIS systems
Secure Processing
Outstanding security and traceability
-
Safety
- Positive identification of samples and reagents
- Triple-detection technology including barometric, capacitive, and colorimetric
-
Traceability
- Bidirectional interfacing with LIS
- Comprehensive event log from sample loading to result release
-
References
1. Burbelo PD et al. (2020). Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. J Infect Dis., jiaa273. Advance online publication. https://doi.org/10.1093/infdis/jiaa273
2. Okba, NMA et al. (2020). Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerging Infectious Diseases, 26(7), 1478-1488. https://dx.doi.org/10.3201/eid2607.200841
3. Sun B et al. (2020). Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):940-948. doi:10.1080/22221751.20 20.1762515
4. Xiang F et al. (2020). Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, ciaa461. Advance online publication. https://doi.org/10.1093/cid/ciaa461
5. Zhao J et al. (2020). Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print, 2020 Mar 28]. Clin Infect Dis. 2020;ciaa344. https://doi.org/10.1093/cid/ciaa344
ScrollOur Best-in-Class
COVID-19 Solutions
Reliable Detection and Differentiation
-
COVID-19 Serology Testing for IVD
As COVID-19 continues to impact countries worldwide, accurate testing is needed to establish serological status of different populations. Bio-Rad now offers the Platelia SARS-CoV-2 Total Ab assay, for the detection of total anti-nucleocapsid antibodies (IgM, IgA, IgG) to SARS-CoV-2. In one test, the assay helps identify the immune response to coronavirus SARS-CoV-2, the virus associated with COVID-19.
The following includes product offerings in certain areas. Some products have limited regional availability. If you have a specific question about our COVID-19 solutions, please contact your local sales office or representative.
Contact a Specialist -
Discover other COVID-19 assay and research solutions from Bio-Rad
- Real-Time PCR instruments and reagents
- SARS-CoV-2 standards
- ddPCR instruments and reagents
- SARS-CoV-2 ddPCR kit (FDA EUA)
-
Total Antibody Detection
The Platelia SARS-CoV-2 Total Ab assay detects total antibodies against SARS-CoV-2, the virus that causes COVID-19, to help identify immune response in individuals as well as provide a more complete understanding of infection rates.
Platelia SARS-CoV-2 Total Ab Assay — At a Glance

Compliant with regulatory standards (US EUA and CE-IVD validated)
COVID-19
Platelia SARS-CoV-2 Total Ab Assay:
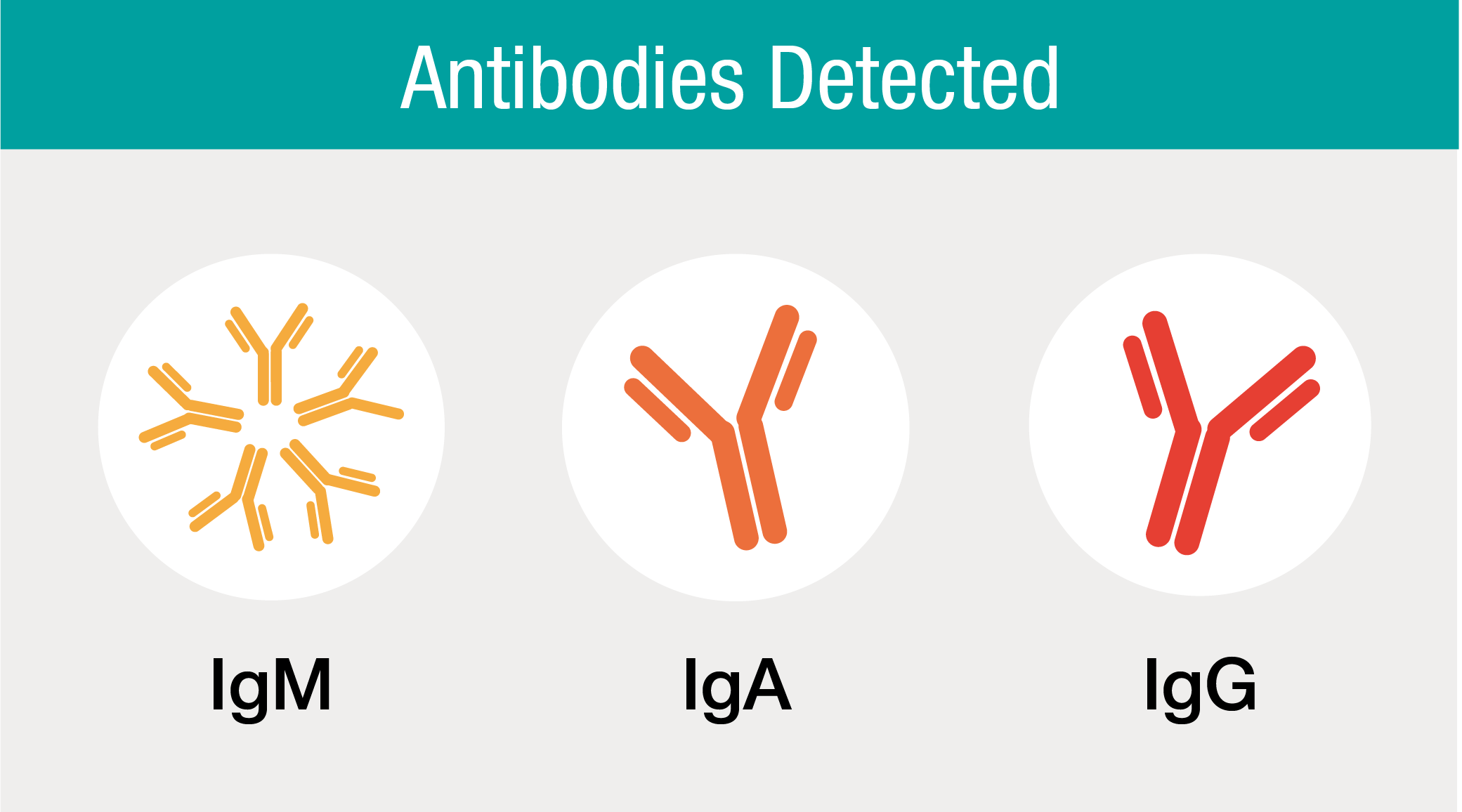
Detection of IgM, IgA and IgG in One Test
The Platelia SARS-CoV-2 Total Ab assay is a high performance screening immunoassay for the detection of anti-nucleocapsid IgM, IgA and IgG antibodies to SARS-CoV-2.
Convenient and Fast
- 1-step antigen capture format (only 90-min incubation time required)
- 96-well microplate testing with breakable strips
- Ready to use with colored reagents
- Visual control of sample dilution and reagent addition
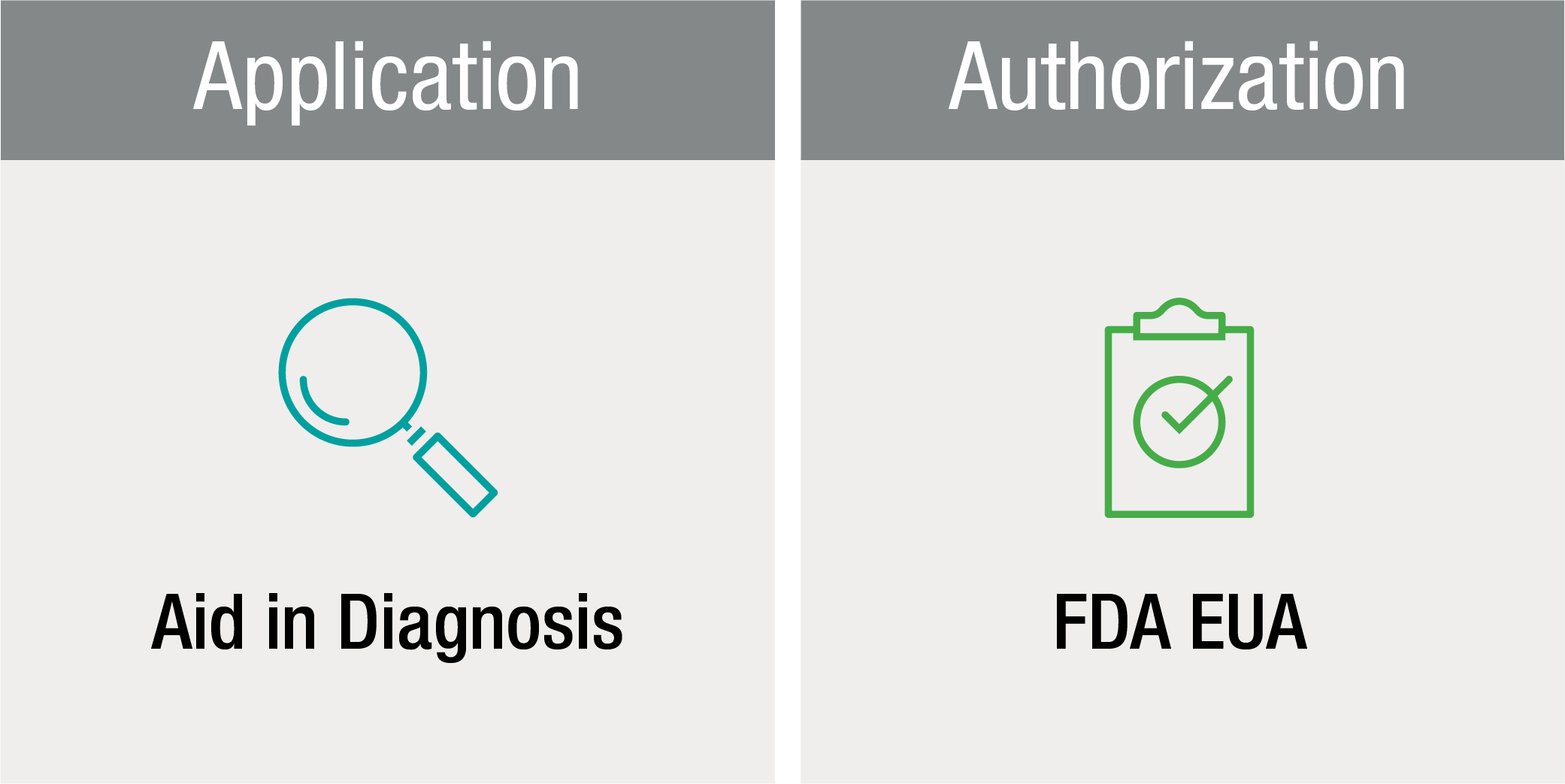
FDA Emergency Use Authorization
In the U.S., this test has been authorized by the FDA under an EUA for use by authorized laboratories. The test has not been FDA cleared or approved. The test has been authorized only for the presence of total antibodies against SARS-CoV-2, not for any other viruses or pathogens. The test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Total Anti-Nucleocapsid Antibody Detection for SARS-CoV-2
The Total Antibody immunoassay format is based on the detection of total antibodies – IgM, IgA and IgG – against the nucleocapsid protein (N-protein) of SARS-CoV-2, all in just one test. The antibodies IgM and IgA are detectable in the case of acute SARS-CoV-2 infection while IgG is detectable in the recovery phase or post infection. Combining the results from these phases delivers a Total Antibody positive result.

Disclaimer: This serological profile is based on projections from other well-known infections and may not fully reflect the course of SARS-CoV-2 infection.

Total Ab Detection vs. Single Ab Detection
Studies by Zhao et al., 2020 demonstrated that total antibody detection with antibodies like IgM, IgA and IgG is associated with better sensitivity, regardless of the time after symptom onset, by comparison to IgM or IgG detection only.

Why Target the Nucleocapsid Protein?
The Nucleocapsid protein (N-protein) is the most abundant SARS-CoV-2 protein and is a significant marker for diagnostic testing assays. Studies in 2020 show that anti-nucleocapsid antibodies (IgM and IgG) can be produced at higher levels and detected very early in the acute infection in comparison to anti-spike antibodies.3,4 ELISA detection of anti-nucleocapsid antibodies is associated with higher sensitivity by comparison to anti-spike antibody detection.1,2

Accurate Performance
High Specificity
The assay showed >99% specificity for specimens from 500 blood donors and 100 hospitalized patients negative for SARS-CoV-2. A total of 600 specimens were tested.
When negative specimens are tested with the Platelia SARS-CoV-2 Total Ab assay, strongly negative values are obtained. The high discrimination between negative and positive values is associated with accuracy and reliability of the results.

No Cross-Reactivity
The assay showed no cross-reactivity to four of the most common coronaviruses, along with EBV, CMV, RSV, flu vaccine and upwards of 25 other medical conditions. A total of 168 specimens were tested.
High Sensitivity
The assay achieved 98% sensitivity overall, with 92% sensitivity for patients tested ≤ 8 days after onset of symptoms and 100% sensitivity for patients tested > 8 days after onset of symptoms. A total of 50 patients were tested.
Thanks to Total Antibody detection, the Platelia SARS-CoV-2 Total Ab assay is associated with earlier detection of seroconversion and consistent detection of the patient’s adaptive immune response over time. With just one test, the Platelia SARS-CoV-2 Total Ab assay reliably detects patients that have been exposed to SARS-CoV-2.

-
Testing on Systems
of Your ChoiceThe Platelia SARS-CoV-2 Total Ab assay is designed for convenient processing on your choice of automated microplate processors. While the assay is recommended for use on the Bio-Rad validated, fully-automated EVOLIS Systems or stand-alone systems (IPS/PR4100/PW40), it can also be run on other validated manual or automated platforms.
-
Bio-Rad EVOLIS Advantages
Validated for Platelia SARS-CoV-2 Total Ab assay testing, Bio-Rad instruments feature automated, scalable processing, full results traceability, and high safety standards.

User-friendly
Intuitive software automates each assay step to reduce operator intervention
Short Time-to-Result
Results obtained in 2 hr 30 min
Optimal Workflow
- Screening of 360 patients in an 8-hour work shift
- Ability to directly load kit reagents
- Possibility to scale up productivity by combining multiple EVOLIS systems
Secure Processing
Outstanding security and traceability
-
Safety
- Positive identification of samples and reagents
- Triple-detection technology including barometric, capacitive, and colorimetric
-
Traceability
- Bidirectional interfacing with LIS
- Comprehensive event log from sample loading to result release
-
References
1. Burbelo PD et al. (2020). Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. J Infect Dis., jiaa273. Advance online publication. https://doi.org/10.1093/infdis/jiaa273
2. Okba, NMA et al. (2020). Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerging Infectious Diseases, 26(7), 1478-1488. https://dx.doi.org/10.3201/eid2607.200841
3. Sun B et al. (2020). Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):940-948. doi:10.1080/22221751.20 20.1762515
4. Xiang F et al. (2020). Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, ciaa461. Advance online publication. https://doi.org/10.1093/cid/ciaa461
5. Zhao J et al. (2020). Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print, 2020 Mar 28]. Clin Infect Dis. 2020;ciaa344. https://doi.org/10.1093/cid/ciaa344
Scroll