The initial step in any blotting experiment is the transfer of proteins from a solution or gel and immobilization of those proteins on a solid membrane support. Immobilization of proteins on a membrane makes the proteins accessible to probes for specific proteins and enables quantitative detection. This section describes the principle of electrophoretic transfer of proteins, and details tank blotting, semi-dry blotting, and microfiltration or dot-blotting methods.
Related Topics: Protein Blotting Equipment, Membranes and Blotting Paper, Transfer Conditions, Protein Blotting Detection and Imaging, Transfer Buffers, and Imaging and Analysis.
Page Contents
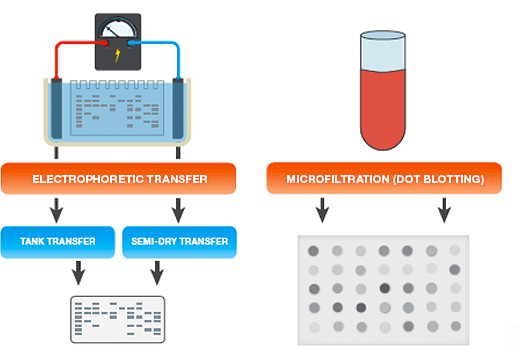
The two most common methods for protein transfer are:
- Electrophoretic transfer — proteins separated in gels (for example, following polyacrylamide gel electrophoresis, or PAGE), are transferred onto membranes by electrophoretic transfer
- Microfiltration (dot blotting) — proteins in solution are bound to membranes by vacuum driven microfiltration. This rapid approach is useful for determining working conditions for a new blotting assay or any other situation where the resolving power of gel electrophoresis is not needed
Electrophoretic Transfer Setup
In electrophoretic transfer, an electric field is used to elute proteins from gels and transfer them to membranes. Electrophoretic transfer is the most widely used blotting method because of its speed and precision in replicating the pattern of separated proteins from a gel to a membrane.
In an electrophoretic transfer, the membrane and protein-containing gel are placed together, with filter paper between two electrodes.
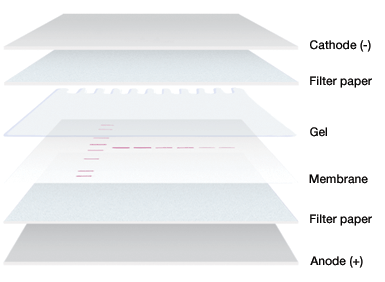
Gel and membrane setup for electrophoretic transfer.
Electrophoretic Transfer Principle
Proteins migrate to the membrane following a current (I) that is generated by applying a voltage (V) across the electrodes* following Ohm's law:
V = I x R
R is the resistance generated by the materials placed between the electrodes (that is, the transfer buffer, gel, membrane, and filter papers).
The electric field strength (E, measured in V/cm) that is generated between the electrodes is the driving force for transfer. Both the applied voltage and the distance between the electrodes then play a major role in governing the rate of elution of the proteins form the gel. A number of other factors, including the size, shape, and charge of the protein*, the pH, viscosity, and ionic strength of the transfer buffer, and the composition of the gel, also influence the elution of particular proteins from gels.
There are practical limits on field strength, however, due to the production of heat during transfer. The heat generated (Joule heating) is proportional to the power consumed by the electrical elements (P), which is equal to the product of the current (I) and voltage (V):
P = I x V = I2 x R
Joule heating increases temperature and decreases resistance of the transfer buffer. Such changes in resistance may lead to inconsistent field strength and transfer, or may cause the transfer buffer to lose its buffering capacity. In addition, excessive heat may cause the gel to deteriorate and stick to the membrane. The major limitation of any electrophoretic transfer method is the ability of the chamber to dissipate heat.
* Proteins denatured with sodium dodecyl sulfate (SDS) carry a net negative charge and migrate toward the anode.
Electrophoretic Transfer Systems
There are two main types of electrophoretic blotting apparatus and transfer procedures (see table below):
- Tank transfer systems — gels and membranes are submerged in transfer buffer in tanks; these systems are useful for most routine protein work, for efficient and quantitative protein transfers, and for transfers of proteins of all sizes. Tank transfer systems offer the most flexibility in choosing voltage settings, blotting times, and cooling options
- Semi-dry systems — gels and membranes are sandwiched between buffer-saturated filter papers that are in direct contact with plate electrodes; these systems are typically easier to set up than tank systems and are useful when high-throughput is necessary and extended transfer times are not required, or when discontinuous buffer systems are used. Active cooling options are limited with semi-dry blotting
In tank blotting systems, the gel and membrane sandwich is entirely submerged under transfer buffer within a buffer tank. A non-conducting cassette holds the membrane in close contact with the gel and the cassette assembly is placed in the tank between the electrodes, transverse to the electrical field, and submerged under conducting transfer buffer (Burnette 1981, Gershoni et al. 1985, Towbin et al. 1979). Although the large volumes of buffer in the tank dissipate the heat generated during transfer and provide the conducting capacity for extended transfer conditions, additional cooling mechanisms are offered by the various tank blotting systems.
In a semi-dry transfer, the gel and membrane are sandwiched between two stacks of filter paper that are in direct contact with plate electrodes (Bjerrum and Schafer-Nielsen 1986, Kyhse-Andersen 1984, Tovey and Baldo 1987). The term semi-dry refers to the limited amount of buffer, which is confined to the two stacks of filter paper.
In semi-dry systems, the distance between the electrodes is limited only by the thickness of the gel and membrane sandwich. As a result, high electric field strengths and high-intensity blotting conditions are achieved. Under semi-dry conditions, some small proteins may be driven through the membrane in response to the high field strengths. Moreover, because low buffer capacity limits run times, some large proteins may be poorly transferred. Use of a discontinuous buffer system, that utilizes different buffer formulations on the anode and the cathode side of the transfer sandwich (see Transfer Buffers) may enhance semi-dry transfer of high molecular weight proteins (>80 kD). As semi-dry transfers require considerably less buffer and are easier to set up than the tank method, laboratories performing large numbers of blots often favor them.
Novel buffer and material formulations have been developed that can be used with higher electric field strengths than those used in typical semi-dry blotting. These conditions yield complete and extremely rapid transfer, with some systems completing transfer in 3–10 min. Such rapid blotting systems do not incorporate external cooling mechanisms, so the high power dissipation may generate more heat than other techniques. Rapid blotting systems are intended for extremely rapid transfers where heat-induced protein denaturation will not affect downstream applications.
Choose the right semi-dry or rapid transfer system based on the demands of your application.
Simple bulk transfer of proteins that are in solution may be achieved by manual application (dotting) to a membrane from a pipet or syringe, or by vacuum-assisted microfiltration. Manual dot-blotting with a pipet or syringe is generally used for small sample volumes. Microfiltration devices, on the other hand, enable application of larger volumes, multiple assays with different probes, and quick, reproducible screening of a large number of samples. Microfiltration facilitates the determination of working conditions for a new blotting assay and is a convenient method in any other situation where the resolving power of gel electrophoresis is not needed.
Comparison of electrophoretic protein transfer systems.
| Tank Blotting | Semi-Dry Blotting | ||
| Traditional | Rapid | ||
| Transfer Time | 30 min–overnight | 15–60 min | 3–10 min |
| Handling convenience | Manual assembly of transfer components | Manual assembly of transfer components | Prepackaged, presaturated components |
| Transfer parameters | Widest range of power settings and transfer times | Power and transfer time limited due to lack of cooling options | Preinstalled, customizable programs for transfer of most proteins, user-programmable settings for traditional semi-dry techniques |
| Molecular weight range | Broad range | Best for 30–120 kD | Broad range |
| Temperature control | Cooling with ice pack or refrigerated water recirculator | None | None |
| Buffer requirement | 1–12 L, system-dependent | 250 ml per blot | No additional buffer required |
Bjerrum OJ and Schafer-Nielsen C (1986). Buffer systems and transfer parameters for semidry electroblotting with a horizontal apparatus. In Electrophoresis '86: Proceedings of the Fifth Meeting of the International Electrophoresis Society, M.J. Dunn, ed. (Weinheim, Germany: Wiley-VCH Verlag GmbH), pp. 315–327.
Burnette WN (1981). "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112, 195–203.
Gershoni JM (1985). Protein blotting: developments and perspectives. Trends Biochem Sci 10, 103–106.
Kyhse-Andersen J (1984). Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 10, 203–209.
Tovey ER and Baldo BA (1987). Comparison of semi-dry and conventional tank-buffer electrotransfer of proteins from polyacrylamide gels to nitrocellulose membranes. Electrophoresis 8, 384–387.
Towbin H et al. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76, 4350–4354.