The first stage of drug development is target discovery, also known as target identification and validation, the process of identifying a potential target for a therapeutic drug for a particular disease or condition that does not have a treatment or for which existing therapeutic agents are lacking in some way. Thus, targets may be known, putative, or novel. Proteins are the most common targets and include enzymes, cell signaling receptors, structural proteins, and regulatory factors such as protein kinases. Other targets that are increasingly being investigated include nucleic acids such as mRNA, lipids, and carbohydrates. Show more
There are two main approaches to drug discovery, phenotype-based and target-based. Recently, there has been a resurgence in phenotype-based screening, which is monitoring changes cells in cells, tissues, or organisms. This type of screening has the ability to identify molecules and pathways that were previously not known to be involved in a disease. In target-based screening, each candidate target is validated by numerous experimental methods including modulation of the target to demonstrate that it is directly or indirectly involved in the disease or condition of interest.
In choosing a target, the probability of it being druggable must also be considered; that is, a drug must be able to bind the target and affect its function in a desirable way. Therefore, the target must be accessible to putative therapeutic agents, and the interaction should produce a measurable biological response. There are a wide range of experimental technologies used for validation, with the validation protocols varying depending on the function and characteristics of the target. Show less
Page Contents
|
|
Gene ExpressionAnalyze gene expression, determine copy number variation (CNV), and initiate early biomarker discovery across a variety of biological samples with our range of validated PCR, qPCR, and Droplet Digital™ PCR instruments and reagents. Explore our large library of disease-specific and pathway-specific predesigned PCR primers, probes, and assay panels that meet MIQE guidelines, or order custom-designed primers and plates. Droplet Digital PCR Systems |
|
|
Protein ExpressionUtilize multiplex immunoassays and our complete western blotting solutions, including gels, imagers, and validated antibodies, in your drug discovery process. Fast, reproducible assessment of proteins with built-in controls and validation steps for protein quantitation streamlines proteomics analysis and monitoring of protein expression levels. Multiplex Cancer Immunoassays |
|
|
Cell AnalysisUse our comprehensive selection of equipment systems, antibodies, assays, and reagents for isolation, characterization, imaging and analysis of both individual cells and populations. Evaluate your studies with our multi-parameter, expandable, easy to use flow cytometers, automated cell sorters and counters, and cell imagers. Cell Imaging |
|
|
AntibodiesDiscover an extensive range of antibodies for applications such as flow cytometry, immunohistochemistry (IHC), western blotting, and ELISA to support your cell-based and proteomics target discovery. Custom services are available to meet your specific target identification and characterization needs Antibodies by Application |
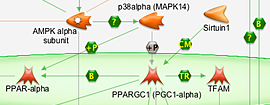
Biochemical and Cellular Assays
In vitro and in vivo discovery approaches with high sample throughput and high-content analysis to characterize biological responses.
Biomarker Discovery & Validation
Biomarker monitoring across drug discovery, development, and clinical stages is key to defining drug effects.
Biologic Development
Biologic development brings patients closer to personalized medicine to treat health problems.
Biosimilar Development
Biosimilar development requires physicochemical and functional characterization to ensure similarity.
Cancer Immunotherapy Development
Learn about trends in cancer immunotherapy development, such as CAR-T, and tools for genomic, cellular, and proteomic research.
CRISPR Gene Editing
CRISPR enables researchers to edit their desired target genes efficiently and accelerate drug development.
Viral Vector Vaccine Development
The COVID-19 pandemic opened the door to expedited vaccine development using new technologies and therapies that have traditionally been used in other applications.