Choosing a Quality Control Product
Choosing the right QC product requires careful consideration. This video covers what laboratory managers should consider when choosing the highest quality control product, including length shelf life, right material for the test system, and other factors. conditions. If your QC plan includes less than optimal QC frequency, ranges, and rules, you may be headed toward unreliable results without warnings.
Featured Content
Learn about the Value of Bio-Rad QC

The Benefits of Independent QC
Quality control (QC) plays an important role in laboratory testing, enabling labs to monitor the analytical quality of a procedure, detect errors, and help ensure the reporting of accurate and reliable patient test results.

Powering Exceptional Labs: The Value of a Bio-Rad Partnership
Quality control (QC) plays a pivotal role in laboratory operations, and effective QC programs are essential for helping ensure reliable outcomes. Learn how Bio-Rad can help identify and support lab's unique needs.
Featured Content
Why Independent QC Matters
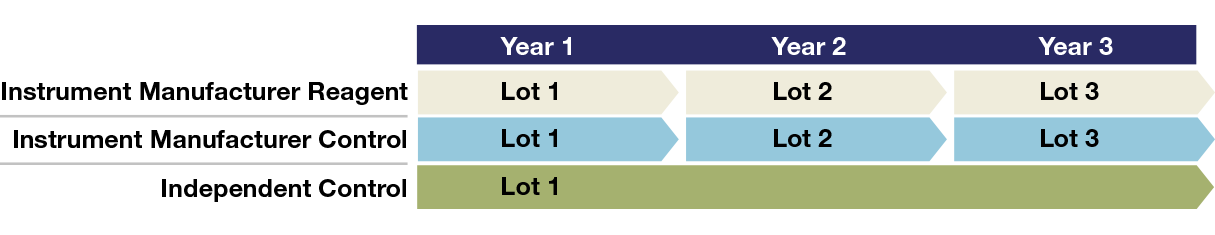
Most regulatory organizations recognize that independent quality controls are an important part of an effective lab QC system. There’s a good reason these guidelines call for independent controls: instrument manufacturer controls are less sensitive to changes in device performance, and analytical errors could affect patient test results. Our unbiased quality controls are not optimized to work with any specific test system and are manufactured independently of instrument, reagents, and calibrators—allowing your lab to manage risk more effectively.
Independent controls are...
Manufactured independently of instrument, reagents, and calibrators
Independent assessment and more sensitive to changes in device performance
Consist of human-based matrix
Similar to patient samples
Offer long shelf lives and lot-to-lot consistency
Long-term QC monitoring and detect shifts that may occur with new reagents and calibrators
Minimize Medically Important Errors
Detect crucial calibration errors
Scenario
Because the manufacturer control was designed to work specifically with the instrument, a lab did not realize that their calibrator material had degraded and patient results were compromised.
Why use independent QC?
Bio-Rad's Liquichek Cardiac Markers Plus Control LT is manufactured independently and therefore more closely mimics patient samples. Using this control would have detected a shift in degradation to avoid erroneous patient results.
Detect important procedural errors
Scenario
During APTT testing, a lab compared results from in-kit controls, which were in range, to an independent Bio-Rad control, which were out of range. This alerted the lab to a missed procedure change.
Why use independent QC?
Bio-Rad's Lyphochek Coagulation Control more closely mimics patient samples. Using this control allowed the lab to detect a procedural error that potentially could have affected patient results
Detect shifts after instrument maintenance
Scenario
After preventative instrument maintenance, the manufacturer controls were within the acceptable range. However, there was a shift for an independent Bio-Rad control. Concern over this shift resulted in the slide reading station needing adjustment.
Why use independent QC?
Bio-Rad's Lyphochek Assayed Chemistry Control found an undetected shift in the recoveries for triglycerides and cholesterol. This shift could have affected patient results.
Detect faulty equipment
Scenario
Results for the instrument manufacturer’s intact PTH control were within acceptable limits. However, an independent Bio-Rad control provided values that were higher than expected. After replacing the luminometer, the Bio-Rad control fell within the acceptable range.
Why use independent QC?
Bio-Rad's Liquichek Specialty Immunoassay Control identified a problem with the luminometer. If it had gone undetected, patient results could have been affected.
Independent QC Training
Featured Content
Experience the Bio-Rad Advantage
Integrated Unity software solutions
By partnering with Bio-Rad, your lab can access the powerful Unity QC data management system, which provides access to the world’s largest peer group through our interlaboratory program. Assessment tools and reports can help your lab meet regulatory and compliance requirements, streamline your QC workflow, create standards for testing and benchmarking lab performance, and report results with confidence.
Highest level of customer service, partnership, and technical support
Bio-Rad can support your lab with more than our leading independent quality controls. We provide QC technical support, program support for both Unity and the QCNet portal, software and connectivity support, and specialized technical proficiency in the form of trained specialists.
Featured Content
Frequently Asked Questions
Featured Content
Laboratory Standards
“Use of independent third-party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer.”
ISO15189: 2012, Subclause 5.6.2. 2 International Organization for Standardization (ISO)samples
"In general, calibrators should not be used as QC materials."
Chemistry and Toxicology Checklist, CHM.14150 CAP Accreditation Program College of American Pathologists (CAP)
"...quality control materials should be different from the calibrator materials to ensure that the QC procedure provides an independent assessment of the measurement procedure’s performance..."
CLSI C24 4th ed., 5.2.5 Relation to Calibrators Clinical and Laboratory Standards Institute (CLSI)
Featured Content
Customer Testimonials
“For a truly independent assessment of the test system, it is very important to use controls that are not provided by the instrument manufacturer.”
Carol Bartlett QC Coordinator, USA
"I trust Bio-Rad controls because they have not been formulated for only one specific test system."
Lab Manager, France
"If you work with manufacturer controls, you will miss a lot of analytical problems that may have an impact on patient results. Moreover, testing independent controls is a requirement of ISO 15189:2012."
J. M. Gras, MD, Clinique Saint-Luc, Bouge, Belgium
Featured Content
Webinars and Videos

Independent QC Benefits
A webinar with Abobaker Mohamed Yagoot, MT (ASCP), MLT (CMLTO) This webinar discusses the benefits of independent QC.
Bio-Rad Independent QC
Bio-Rad independent QC helps increase consistency of your system, reducing risk of erroneous test results.