QC Data Management Learning

Is It Time to Upgrade Your QC Testing Data Management Systems? Key Questions to Consider
QC Data management plays a crucial role in the effectiveness of QC testing. A powerful data management solution has the ability to develop effective QC strategies and manage risks.

How the Right QC Provider Can Help You Meet Accreditation Requirements
Bio-Rad Laboratories provides a wide range of simple, effective solutions to help meet general regulatory/ accreditation standards and provide greater confidence in the laboratory’s performance.

The Value of the Unity QC Peer Reporting Program
A laboratory’s value is only as good as its documented performance. Analytically capturing precision, reliability, and efficiency is crucial to properly evaluate your Quality Control (QC) system. Learn how the Unity QC Peer Program helps labs quickly identify trends and troubleshoot QC errors.

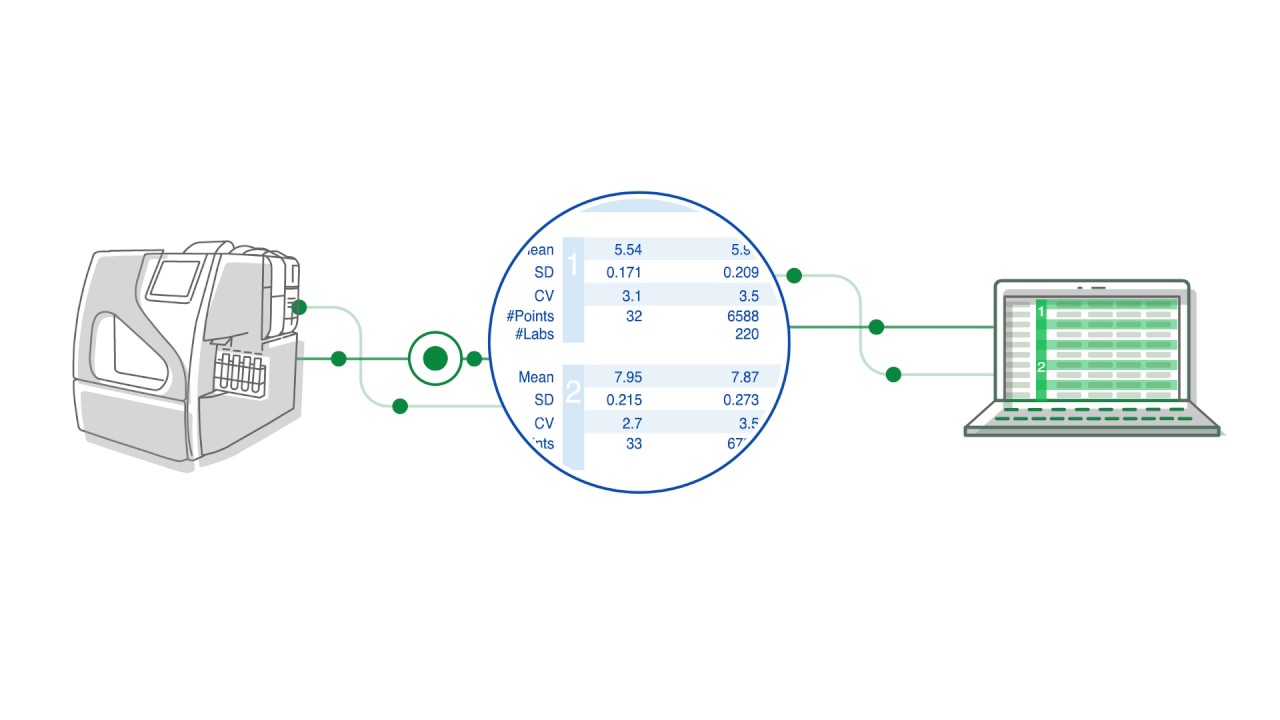
How Unity Data Management Solutions Can Help You Manage a Better Lab
Managing a successful clinical diagnostic lab depends on multiple variables: sufficient budget, well-trained staff, time and resources, reliability of results, and compliance with regulatory standards. Learn how Unity can help optimize your lab to reach performance goals.

A Peer Comparison Tool for QC Performance
Explore the latest features from Unity Next Peer QC. See how you can meet compliance inspections and compare QC results with peers in one location.

Did You Know:
”Did You Know?” guides current Unity Real Time labs through in-demand software features. Learn about hidden functionalities and shortcuts to better manage your lab.

Unity Next Peer QC
Learn how to troubleshoot QC errors and increase chances of passing a proficiency test with Unity Next Peer QC.
Unity Training Videos

Whether you need help configuring our Unity data management software to fit your lab’s designs and goals, or you require training on how to use Unity’s basic or advanced solutions, we offer a broad range of educational and training videos and other resources.
Unity Real Time Training
Six Unity Real Time training videos provide an introduction to the system, setup, data entry, data evaluation, peer group comparison, and measurement uncertainty reporting.
Unity Real Time Online demo
Unity Real Time Online is a web-based expert QC data management solution that facilitates regulatory compliance under CLIA and ISO 15189.
Featured Content
QC Design and Strategy
We offer three levels of training around QC design and strategy. Whether you’re looking for the basics or a lesson in how to participate in our Unity Interlaboratory Program, these resources can help optimize your knowledge to build a better lab.
Articles
- Repeating control and patient samples
- Quality Control in the Evolving Landscape of Molecular Diagnostics Testing
- 5 Things You Need to Know about LOINC
- Digital management of quality control a critical tool for the modern lab
- Optimize Quality Control Data Management with Bio-Rad’s Unity Real Time Software
- Scripted Quality Control
Featured Content
QCLive Training Modules

Attend a live, virtual training session with one of Bio-Rad’s professional trainers—and build your skills in efficient QC data management. Sessions are complimentary, presented in English, and available to worldwide customers. P.A.C.E. credits (CEU) are offered.
Unity Interlaboratory Reports
Learn about the reports that are included with your lab’s participation in the Unity Interlaboratory Program, the world’s largest QC peer group program.
Analytical Goals
Learn how to use Analytical Goals in Unity Real Time 2 as a retrospective or ongoing review tool that can help you optimize QC effort and cost.
Bench Review
Learn basic navigation of the Unity Real Time 2 software, how to enter QC results manually, and how to use the Bench/Supervisor Review tools to perform QC evaluation and documentation.
Data Analysis Grid
Learn about a feature that helps you perform unlimited types of data comparisons in Unity Real Time 2. Use the Data analysis grid to compare your data against the peer group, against your evaluation means/standard deviation, and against multiple instruments.
Advanced Charts
Learn how the Yundt and Youden charts available with Unity Real Time 2 and Unity Real Time online can help you evaluate bias, imprecision, and linearity.
Featured Content
Technical Documents
Unity Alert
LinearityWeb
Lotes en reserva
Featured Content
Webinars
Bio-Rad ADLM Industry Workshop
From the Bio-Rad ADLM industry workshop, “Embracing New Quality Specifications: Adjusting Lab Practices - CLIA 2024 and Beyond” check out the three featured presentations, comprising of leading clinical diagnostics professionals sharing their key understandings into the implications of changing quality specifications using the new CLIA 2024 Proficiency Testing (PT) limits as an example. Learn to address the critical steps in QC troubleshooting, the necessity of revising risk assessment with new quality specifications and the value of interlaboratory peer programs in preparing for CLIA 2024 PT. Watch now to learn more.

Evaluating the Impact of an Out-of-Control Event
Lorin Bachmann, PhD, DABCC
Discover how to effectively recover from out-of-control events in the lab with a detailed overview of evaluating the impact on patient results plus outlining approaches for preventative action if a lab’s QC program is insufficient or if quality specifications have changed.

Risk Management of Changing Quality Specifications
James H. Nichols, PhD, DABCC, FADLM
Evaluate and assess the quality of laboratory tests and identify the impact of changes to CLIA Proficiency Testing (PT) criteria while considering the risk of changing quality specifications on lab performance.

Predicting CLIA 2024 PT Perf. With an Interlab. Peer Comparison Program
John Yundt-Pacheco, MSCS
Explore the structure of the CLIA 2024 PT program. Learn how to compute PT performance metrics with interlaboratory peer group programs and see how to use risk contour plots to evaluate the impact of changing the lab mean and SD.