On This Page |
A Microbial Immune System | A Gene Editing System | Applications of CRISPR Technology | Classroom Activities | References |
On This Page |
A Microbial Immune System | A Gene Editing System | Applications of CRISPR Technology | Classroom Activities | References | CRISPR for the Classroom |
CRISPR-Cas — A Microbial Immune System
Humans have complex immune systems that involve the coordinated activities of multiple cell types, organs, and signaling systems to recognize and respond to active infections. Prokaryotes — bacteria and archaea — also have a form of adaptive immunity that allows them to recognize and respond to viral infections.

Bacterial Chromosome A CRISPR region within a microbial genome.
Some prokaryotic genomes contain short, palindromic DNA sequences that are repeated many times, with unique "spacer" sequences between the repeats. These "clustered regularly interspaced short palindromic repeats" (CRISPR) are followed by short segments of spacer DNA that match DNA sequences found in bacteriophage genomes (bacteriophage are viruses known to infect bacteria). Groupings of CRISPR-associated (Cas) genes are also found next to CRISPR sequences, and these Cas genes encode enzymes that cut DNA in specific places, like precise molecular scissors.
CRISPR-Cas9 sequences work together to provide an immune response in which a microbe could recognize invading DNA and unleash Cas enzymes to cut and disarm it. CRISPR sequences and the Cas enzymes are the keys to the microbial "adaptive immune response," which involves three phases:
- Cutting and Capture — When bacteria are infected by a virus, they use their CRISPR system to cut up the invading viral DNA and insert pieces of it (spacers) into their own genome as a "memory" of the infection
- Monitoring — Bacteria transcribe the spacers into RNA, which can form a complex with the Cas9 enzyme. These complexes monitor the cell for any DNA sequence complementary to the RNA
- Defense — If matching (viral) DNA is encountered, the spacer RNA-Cas9 complex binds to it and cuts the viral DNA to prevent it from replicating. This halts the viral infection
Using this system, bacteria can collect sequences from many different infecting viruses to create a "library." Since the CRISPR sequence is contained in genomic DNA, it is passed on to each generation, and the library continues to change and adapt to more common threats over time.

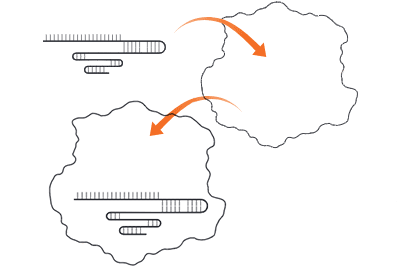
Acquire Foreign DNA Sequences 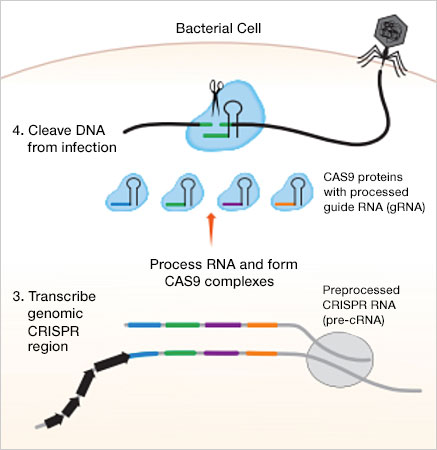
Defend Against Future Infection
The CRISPR-Cas9 microbial defense system. 1. The Cas1-Cas2 enzymes of the microbe recognize and cut out a segment of foreign DNA. 2. The Cas1-Cas2 enzymes insert the DNA segment into the CRISPR region of the bacterial genome as a spacer. 3. A spacer sequence is transcribed and then linked to a Cas9 protein. 4. Upon reinfection by the same invader, the CRISPR-Cas9 complex can recognize the foreign DNA sequence and cut it to prevent complete reinfection.
CRISPR-Cas9 — A Gene Editing System
The CRISPR-Cas9 system uses modified components of the bacterial CRISPR system to direct target-specific cutting of double-stranded DNA. DNA repair mechanisms then take over to fix the break in a manner that modifies the genetic sequence that has been cut.
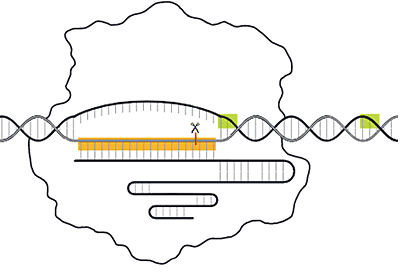
Cutting the DNA
- Cas9 enzyme (Cas9) — an endonuclease that cuts both strands of DNA at a specific site. Multiple types of Cas enzymes are found in nature, but Cas9 is commonly used in the laboratory
- Single guide RNA (sgRNA) — an engineered RNA that forms a complex with Cas9. The sgRNA is a fusion of two regions that occur as separate RNAs in nature:
- Guiding region — part of the CRISPR RNA or crRNA in nature, a 20-nucleotide region that is complementary to the target region and defines the target DNA sequence that Cas9 cuts. Scientists customize this sequence for their own targets
- Scaffold region — the trans-activating CRISPR RNA or tracrRNA in nature, this region forms a multi-hairpin loop structure (scaffold) that binds in a crevice of the Cas9 protein
- Protospacer adjacent motif (PAM) — required for Cas9 function, this sequence motif is immediately downstream of the target sequence. Cas9 recognizes the PAM sequence 5’-NGG, where N can be any nucleotide (A, T, C, or G). When Cas9 binds the PAM, it separates the DNA strands of the adjacent sequence to allow binding of the sgRNA. If the sgRNA is complementary to that sequence, Cas9 cuts the DNA
5 Steps of Cas9 DNA Cleavage
Repairing the Break to Engineer the Change
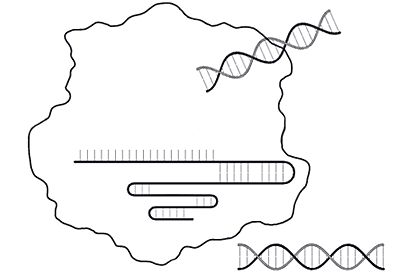
Researchers can use the cell’s own DNA repair machinery to modify, insert, or delete a nucleotide sequence. The repair can happen in two ways:
- Non-homologous end joining (NHEJ) — enzymes reconnect the ends of the double-stranded break back together. This process may randomly insert or delete one or more bases and can cause mutations that can disrupt gene function or expression
- Homology directed repair (HDR) — proteins patch the break using donor template DNA. Researchers design the donor template DNA that may include a desired sequence flanked on both sides by "homology arms" that match the sequence upstream and downstream of the cut. A complementary DNA strand is created during the repair
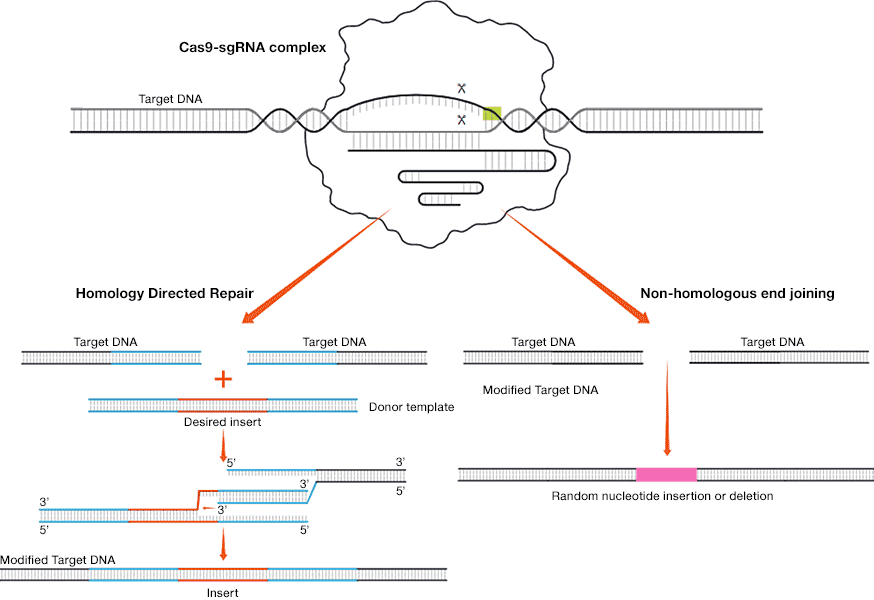
DNA repair via homology directed repair and non-homologous end joining (Scroll right to view image)
Classroom Activities and Resources

YouTube CRISPR Gene Editing Playlist
Have your students gain perspectives about CRISPR from some of the industry’s leading experts.
Classroom Activities and Resources
CRISPR Paper Model Activity (PDF 675 KB)
Have your students use this CRISPR paper model activity at home to learn about the CRISPR-Cas9 system.
Bioinformatics Activity (PDF 6.1 MB)
Students can use this activity to design Cas9 target sites in the human genome and determine risk for off-target effect.
PowerPoint Presentation for Classroom Use (PPT 14.9 MB)
Use and modify this slide deck to help guide your students through the CRISPR-Cas9 gene editing lab activity.
Gene Editing and CRISPR References
- Berry MH et al. (2019) Restoration of high-sensitivity and adapting vision with a cone opsin. Nature Communications 10, 1221.
- Carroll D (2017) Genome editing: past, present, and future. Yale J Biol Med 90, 653–659.
- Cong L et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823.
- Cribbs A et al. (2017) Science and bioethics of CRISPR-Cas9 gene editing: an analysis towards separating facts and fiction. Yale J Biol Med 90, 625–634.
- Goodyear, D (2023) The transformative, alarming power of gene editing, The New Yorker.
- Jinek M et al. (2012) A programmable dual RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821.
- Park A (2016) A new technique that lets scientists edit DNA is transforming science — and raising difficult questions. Time 43–48.
- Thurtle‐Schmidt D and Lo T (2018) Molecular biology at the cutting edge: A review on CRISPR/CAS9 gene editing for undergraduates. Biochem Mol Biol Educ 46, 195-205.
- Wang JY and Doudna JA (2023) CRISPR technology: a decade of genome editing Is only the beginning. Science 379, eadd8643.