
On This Page |
Why ddPCR technology | Why Bio-Rad | QX ONE ddPCR System | Viral Titer ddPCR Assays | Resources |
Experience the Proven Accuracy and Reproducibility of ddPCR™ Technology
When you are ready to switch to ddPCR™ technology for viral titer determination or to upgrade to an easy-to-use, high-performance ddPCR platform for your gene therapy workflows, Bio-Rad is the proven choice.
Why Use Droplet Digital™ PCR for Viral Titer Determination?
Whether you are in the early stages of gene therapy bioprocess development, scaling upstream or downstream production, or running assays for QC release, measuring viral titer is key. Understanding how many individual viral genomes are present per milliliter of solution is one of several measurements of the potency of your therapeutic and a critical quality attribute during bioprocess development.
qPCR Is Neither Accurate Nor Precise Enough for Gene Therapy Applications
For several years, qPCR was the go-to technology for viral titer determination, as it is often the method of choice for nucleic acid quantitation. However, qPCR is neither accurate nor precise enough for gene therapy applications.1 Quantification with qPCR is dependent on amplification efficiency, which can be affected by a few factors including:
- Suboptimal design of the primers and probes
- Inhibitors, whose concentration may vary from sample-to-sample
- Secondary structure in the template
In addition, qPCR requires the generation of a standard curve, which introduces the risk of inaccuracy if the calibration is incorrect.1
Learn more about the use of ddPCR for AAV titer determination in Dobnik, et al.,1
"Results of the analyses showed that Droplet Digital™ PCR (ddPCR™) performs better than quantitative real-time PCR (qPCR), in terms of robustness and assay variance, and this was especially relevant for partially purified (in-process) samples."1
ddPCR Is a Superior Technology for Viral Titer Determination for Gene Therapy
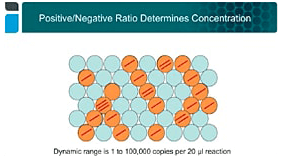
In contrast to viral titer measurement with qPCR, quantification with ddPCR technology does not depend on amplification efficiency but rather is an endpoint PCR assay that delivers absolute quantitation. As a result, measurement with ddPCR technology is more precise and accurate than qPCR, and with Bio-Rad’s family of ddPCR instruments, viral titer determination using ddPCR is straightforward to learn and implement.
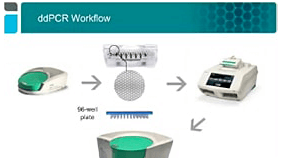
Quantification using ddPCR technology occurs by first separating sample viral genomes into discrete, volumetrically defined droplet partitions, amplifying the nucleic acid within the partitions, and determining the ratio of sample-positive partitions to sample-negative partitions.

Introduction to Droplet Digital PCR: Workflow and Applications
This webinar introduces the concept, workflow, and performance of Droplet Digital PCR and highlights the diverse applications showcased in recent journal publications.

Introduction to Digital PCR
Discover the basics and fundamentals of digital PCR technology in our Learning Center.

Did you know?
Bridging studies to transition from qPCR to ddPCR are straightforward and easy to design and run.
Why Bio-Rad? Our Proven ddPCR Performance in Gene Therapy Applications
With 10+ years of experience developing and manufacturing an innovative ddPCR technology and 83+ peer-reviewed publications that use a Bio-Rad ddPCR solution for gene therapy research, development, and manufacturing, Bio-Rad is the proven supplier for ddPCR instruments and assays for gene therapy applications.
- Regulatory edition software to assist with U.S. FDA 21 CFR Part 11 compliance
- Installation qualification/operational qualification (IQ/OQ) service
- Premier service offerings with rapid on-site support
Increase Throughput with the QX ONE ddPCR System

The QX ONE ddPCR System is designed to offer walk-away nucleic acid quantification. The system fully automates the ddPCR workflow, from droplet generation to nucleic acid amplification to droplet reading. The QX ONE System has multiplex capabilities with 4 channels that enable processing of 480 samples without user intervention.
Learn More about the QX ONE ddPCR System »
QX ONE Droplet Digital PCR System Tour
The QX ONE ddPCR System seamlessly integrates a standard ddPCR workflow of droplet generation, thermal cycling, droplet reading, and analysis into a hands-free precision platform.
ddPCR Assays for AAV Titer Determination and More
Bio-Rad's Droplet Digital PCR (ddPCR) Assays for AAV titer determination utilize the precision and sensitivity of ddPCR technology to deliver an absolute count of AAV vector genome copies in your sample. These assays can be ordered through the Cell and Gene Therapy Design Engine and come in two varieties:
- Custom ddPCR Cell and Gene Therapy Assays generated for target gene of interest
- Ready-to-order ddPCR Expert Design Assays for common viral vector and plasmid
elements
Resources
Webinars
Introduction to Droplet Digital PCR: Workflow and Applications
Learn the concept, workflow, and diverse applications of ddPCR technology showcased in recent journal publications.
Droplet Digital PCR Tips and Tricks: ddPCR Assay Design
Find tips and tricks used in ddPCR assay design. Topics covered include the availability of commercial ddPCR assays.
Publications
Systemic Treatment of Fabry Disease Using a Novel AAV9 Vector Expressing α-Galactosidase A
Biferi MG, et al. Mol Ther Methods Clin Dev. 2020 Oct 22;20:1-17. doi: 10.1016/j.omtm.2020.10.016
Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae
Omichi R, et al. Mol Ther Methods Clin Dev. 2020 May 13;17:1167-1177. doi: 10.1016/j.omtm.2020.05.007
Documents

Harnessing Analytical Technologies to Modify Your AAV Development Workflow
Discover how the latest analytical tools can achieve different assays with high reproducibility and accuracy.

Developing Safe and Effective AAV-Based Gene Therapies Using ddPCR™ Technology
Learn how ddPCR technology offers the accuracy to quantify AAV viral titers to support many aspects of quality control for AAV-based gene therapy development.

Measuring AAV Vector Genome Titer Using Droplet Digital™ PCR Protocol
Get started with this protocol designed to measure AAV vector genome titer using ddPCR technology for both in-process and purified samples.

Transitioning Your Assay from Quantitative PCR to Droplet Digital PCR
Learn how transitioning your assay from qPCR to ddPCR can be simple and provide consistent results.

ddPCR Assays for Adeno-Associated Virus (AAV) Vector Genome Titer
Discover the complete ddPCR workflow solution for absolute quantification of AAV vector genome copies.

ddPCR Viral Quantification: Success Story
Read this success story to learn how ddPCR technology represents a highly precise method for determining AAV vector genome titers.
References
- Dobnik D, Kogovšek P, Jakomin T, et al. Accurate Quantification and Characterization of Adeno-Associated Viral Vectors. Front Microbiol. 2019;10. Accessed May 22, 2022. https://www.frontiersin.org/article/10.3389/fmicb.2019.01570
Discover More Gene Therapy Development Solutions
-
Gene Therapy Development Overview
Bring efficiency to your workflows and confidence to your decision making with our gene therapy manufacturing products.
-

Viral Vector Characterization
Enable efficient and accurate characterization of your viral vector with our full workflow of products, whether your gene therapy uses AAV or another vector technology.
-

Viral Vector QC
Harness the power of ddPCR for accurate and precise detection and quantification of host cell DNA and pathogenic microbes.
