Selection of the appropriate primary and secondary antibodies is crucial for effective western blotting. This section briefly describes the general structure of antibodies and provides some tips for proper dilution and selection of primary and secondary antibodies.
Related Topics: Detection Methods for Western Blotting, Stripping and Reprobing Membranes, Western Blotting.
Page Contents
An antibody is an immunoglobulin protein such as IgG that is synthesized by an animal in response to exposure to a foreign substance, or antigen. Antibodies have specific affinity for the antigens that elicited their synthesis. Structurally, most IgG class antibodies contain four polypeptide chains (two identical heavy chains of ~55 kD and two identical light chains of ~25 kD) oriented in a "Y" shape. These are held together by disulfide bridges and noncovalent interactions. These proteins contain an Fab region with specific affinity for the antigens that elicited their synthesis. In addition, a constant region (Fc) on the antibody provides binding sites for proteins needed during an immune response.
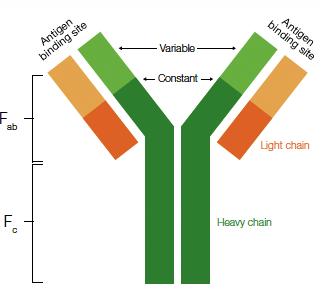
Antibody structure. The components of a typical IgG molecule are highlighted and include the Fab fragment containing the variable region responsible for antigen binding and the Fc, constant region, necessary for binding other proteins involved in the immune response.
The primary antibody recognizes and binds to the target antigen on the membrane. For successful incubations with primary antibodies, the entire blot must be covered with antibody-containing solution. The optimum antibody concentration is the greatest dilution of antibody that still yields a strong positive signal without background or nonspecific reactions. Instructions for antibodies obtained from a manufacturer typically suggest a starting dilution range. For custom antibodies, or those where a dilution range is not suggested, good starting points are:
- 1:100–1:1,000 dilution of the primary antibody in buffer when serum or tissue culture supernatants are the source of the primary antibody
- 1:500–1:10,000 dilution of chromatographically purified monospecific antibodies
- 1:1,000–1:100,000 dilution may be used when ascites fluid is the source of antibody
For each new lot of primary antibody, determine the appropriate concentration or dilution (titer) empirically. The Mini-PROTEAN® II multiscreen apparatus and mini incubation trays are useful tools for determining antibody titer.
Secondary antibodies are specific for the isotype (class) and the species of the primary antibody (for example, a goat anti-rabbit secondary antibody is an antibody generated in goat for detection of a primary antibody generated in a rabbit). Secondary antibodies bind to multiple sites on primary antibodies to increase detection sensitivity. For immunodetection, only blotting-grade species-specific secondary antibodies are used.
Secondary antibodies can be labeled and detected in a variety of ways. The antibody can be radiolabeled or linked to a fluorescent compound or to gold particles, but most commonly, the antibody is conjugated to an enzyme, such as alkaline phosphatase (AP) or horseradish peroxidase (HRP). If the secondary antibody is biotinylated, biotin-avidin-AP or -HRP complexes can be formed. Addition of a suitable enzyme substrate results in production of a colored precipitate or fluorescent or chemiluminescent product through dephosphorylation (by AP) or oxidation (by HRP).
Since the purity of the reagents is critical to the success of the experiment, the following steps are critical if the antibodies used are not blotting-grade:
- Purify all sera by affinity chromatography to obtain only those antibodies directed against the particular IgG; otherwise, background staining and false positive reactions due to nonspecific antibody binding may occur
- Cross-absorb the purified antibody solution against an unrelated species; for example, human IgG for anti-rabbit and anti-mouse antibodies, and bovine IgG for anti-human reagents, to remove antibodies that are not specific for the species of interest
Blotting-grade antibodies are directed to both heavy and light chains of the IgG molecules, so the reagents can be used to identify other classes and subtypes of immunoglobulins.
Protein A and protein G are bacterial cell surface proteins that bind to the Fc regions of immunoglobulin molecules (Akerstrom et al. 1985, Boyle and Reis 1987, Goding 1978, Langone 1982). The advantage of using protein A or protein G is their ability to bind to antibodies of many different species (see Table below). This is often desirable for laboratories using antibody probes from many different species or for those using one of the less common primary antibody systems (rat, goat, or guinea pig) in their experiments. In addition, these conjugates bind only to antibody molecules; this can reduce the background from nonspecific binding of antibodies to membrane-bound proteins when a low-titer, poorly purified second antibody is used.
The major limitation of protein A and protein G conjugates is their lower sensitivity. Because only one ligand molecule binds to each antibody, the enhancement of a multiple-binding detection system, such as a species-specific polyclonal antibody, is lost. Generally, the species-specific antibody is 10–50 times more sensitive than the ligand reagent when the same detection system is used.
Immunoglobulin-binding specificities of protein A and protein G.
| Immunoglobulin | Protein A | Protein G |
| Human IgG1 | •• | •• |
| Human IgG2 | •• | •• |
| Human IgG3 | — | •• |
| Human IgG4 | •• | •• |
| Mouse IgG1 | • | • |
| Mouse IgG2 | •• | •• |
| Mouse IgG3 | •• | •• |
| Mouse IgG4 | •• | •• |
| Rat IgG1 | • | • |
| Rat IgG2a | — | •• |
| Rat IgG2b | •• | • |
| Rat IgG2c | •• | •• |
| Pig IgG | • | •• |
| Rabbit IgG | •• | •• |
| Bovine IgG1 | — | •• |
| Bovine IgG2 | •• | •• |
| Sheep IgG1 | — | •• |
| Sheep IgG2 | • | •• |
| Goat IgG1 | • | •• |
| Goat IgG2 | •• | •• |
| Horse IgG(ab) | • | •• |
| Horse IgG(c) | • | •• |
| Horse IgG(t) | — | • |
| Dog IgG | •• | • |
| •• = Strong binding • = Weak binding — = No binding | ||
In some experiments, protein blots must be screened for a number of different antigens or under a number of different conditions. The Mini-PROTEAN II multiscreen apparatus and mini incubation trays are useful tools for multiscreening purposes.

Akerstrom B et al. (1985). Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol 135, 2589–2592.
Boyle MDP and Reis KJ (1987). Bacterial Fc receptors. Biotechnology 5, 697–703.
Goding JW (1978). Use of staphylococcal protein A as an immunological reagent. J Immunol Methods 20, 241–253.
Langone JJ (1982). Use of labeled protein A in quantitative immunochemical analysis of antigens and antibodies. J Immunol Methods 51, 3–22.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6216 | Detection Buffer Formulations | Click to download |
| 6218 | Blot Stripping & Reprobing | Click to download |
| 6219 | Immunodetection | Click to download |
