
Overview
TORCH testing aids in the detection of Toxoplasma gondii, rubella, cytomegalovirus, and herpes simplex virus. Testing patients before pregnancy or during the prenatal period determines the immune status of the mother and identifies infections early. While TORCH infections may not cause significant illness in healthy adults, they can lead to congenital infections and other adverse outcomes to newborns if transmitted to a fetus during pregnancy.
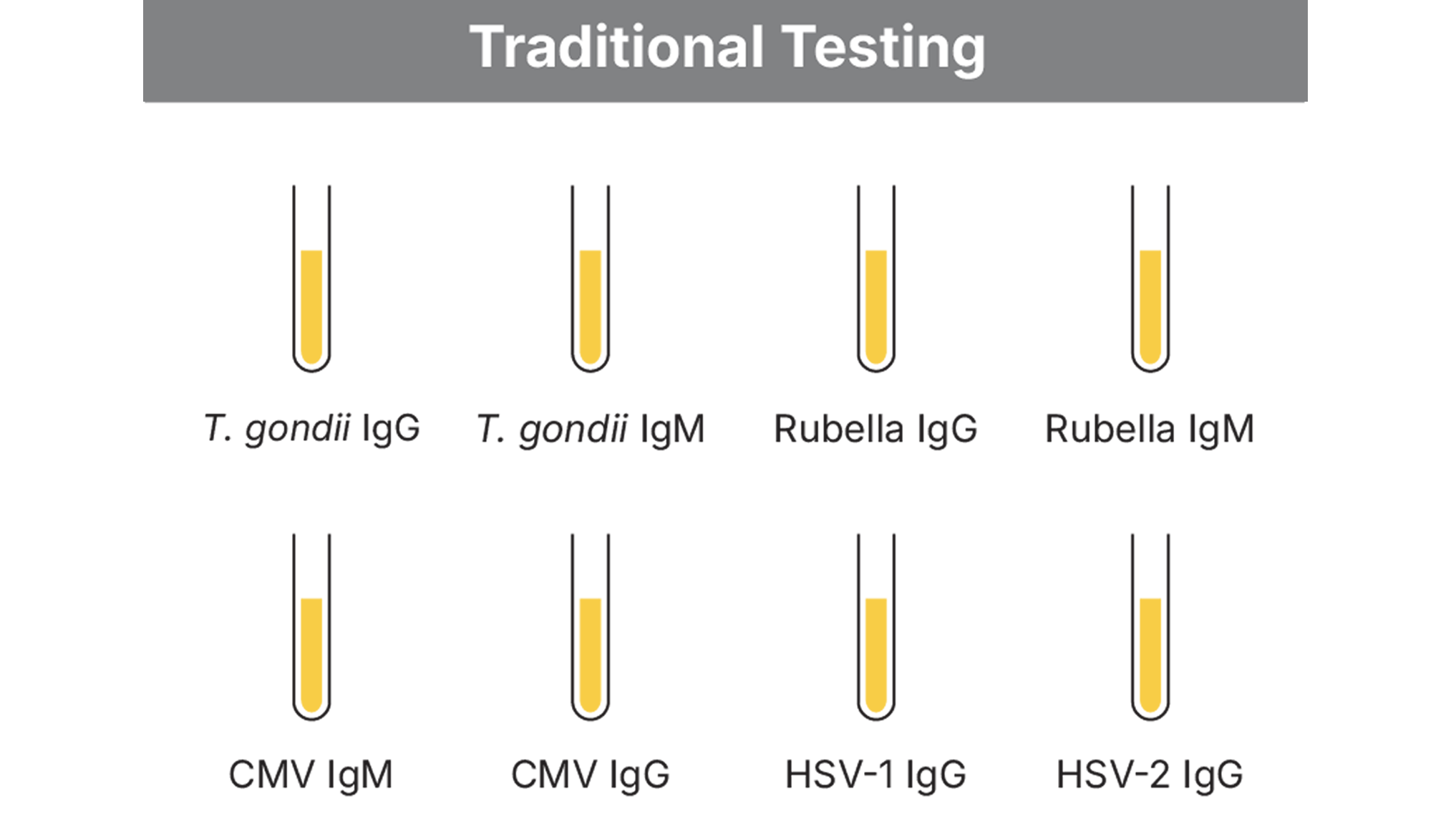
Clinical laboratories often face the challenge of managing the array of different tests required for prenatal testing panels. This can create workflow inefficiencies and add manual processes that burden limited laboratory resources and increase testing costs.
The Power of Multiplex TORCH Testing
Through multiplexing, a laboratory can quickly test for immune status against the most common congenital infections, reducing the risk of transmission.

Toxoplasma gondii IgG
Rubella IgG
Cytomegalovirus IgG
Toxoplasma gondii IgM
Rubella IgM
Cytomegalovirus IgM
Herpes simplex virus type 1 IgG
Herpes simplex virus type 2 IgG
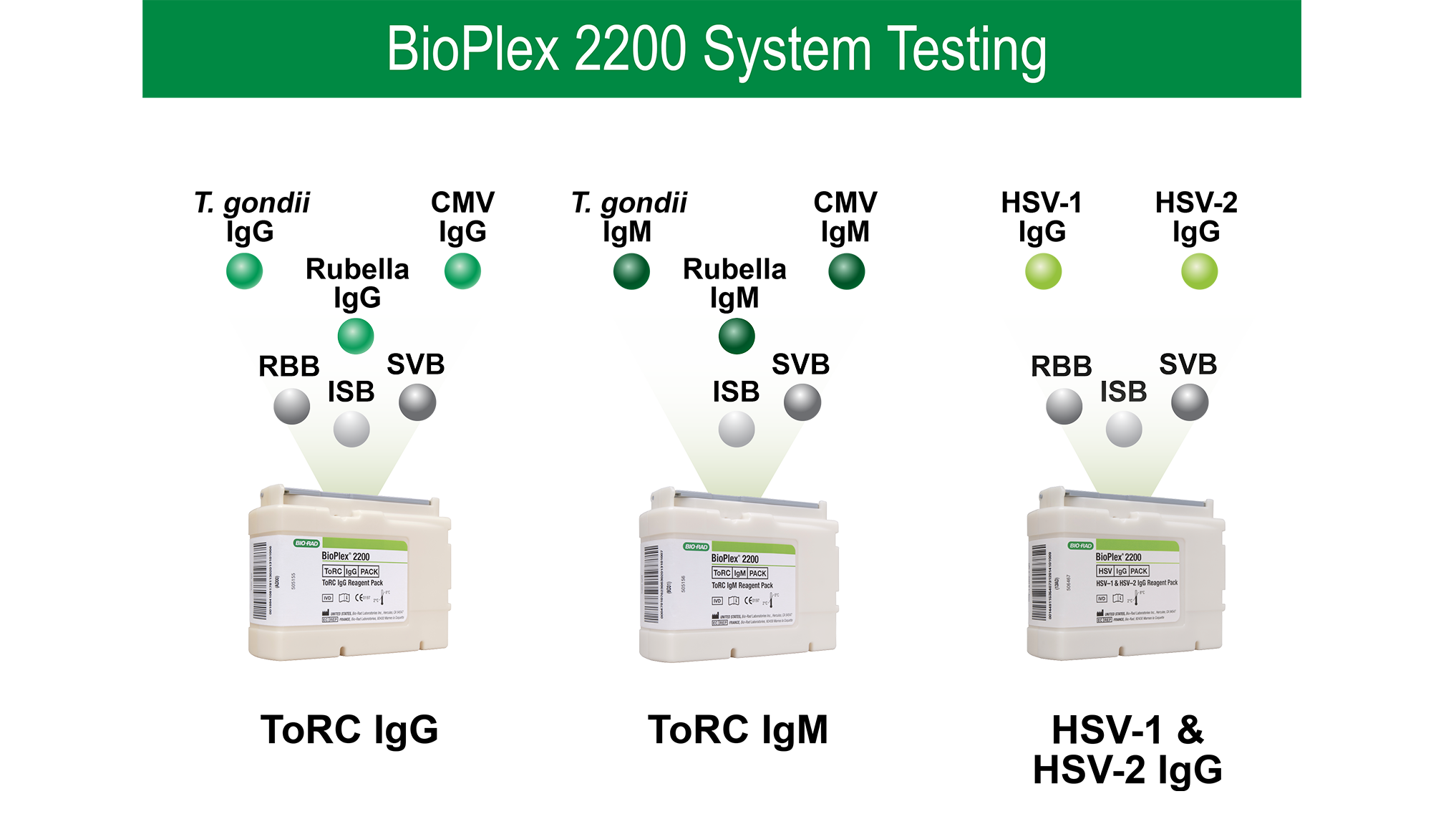
Multiplex Automation That Works as Hard as You Do
The BioPlex 2200 System uses multiplex technology to deliver more results with fewer tests.
Efficiency of Multiplex Testing
Transform your lab operations with the advantages of multiplex testing:

- Generate multiple results in a single test
- Consolidate test methods on a fully automated, random-access multiplex platform
- Eliminate manual and batch testing
- Reduce laboratory waste and optimize reagent storage
- Decrease send-out testing to improve turnaround time
Confident Detection of Congenital Infections
Patient care is at the heart of what you do. The BioPlex 2200 TORCH panels deliver dependable results that help guide clinical choices and provide the best care for your patients.
Reliable Results for Confident Clinical Decisions
The BioPlex 2200 ToRC IgG, ToRC IgM, and HSV-1 & HSV-2 IgG panels help ensure reliable outcomes through:

- Verification of assay performance and result integrity in every test
- Simplified workflow with full automation
- Ready-to-use reagents, calibrators, and controls to minimize errors
- Reliability in patient results driven by strong assay precision
- Strong agreement with CDC disease-specific panels and quantitative test results correlated to WHO standards
Every Result Matters—For You and Your Patients
In addition to external independent quality controls, patented quality control beads are integrated into the BioPlex 2200 ToRC IgG, ToRC IgM, and HSV-1 & HSV-2 IgG panels. This enables the real-time analysis of assay parameters for every test, providing additional confidence in every patient result.

Fewer Tests. Lower Costs. Greater VaIue.
The BioPlex 2200 ToRC IgG, ToRC IgM, and HSV-1 & HSV-2 IgG panels can help your lab optimize testing processes and minimize costs.
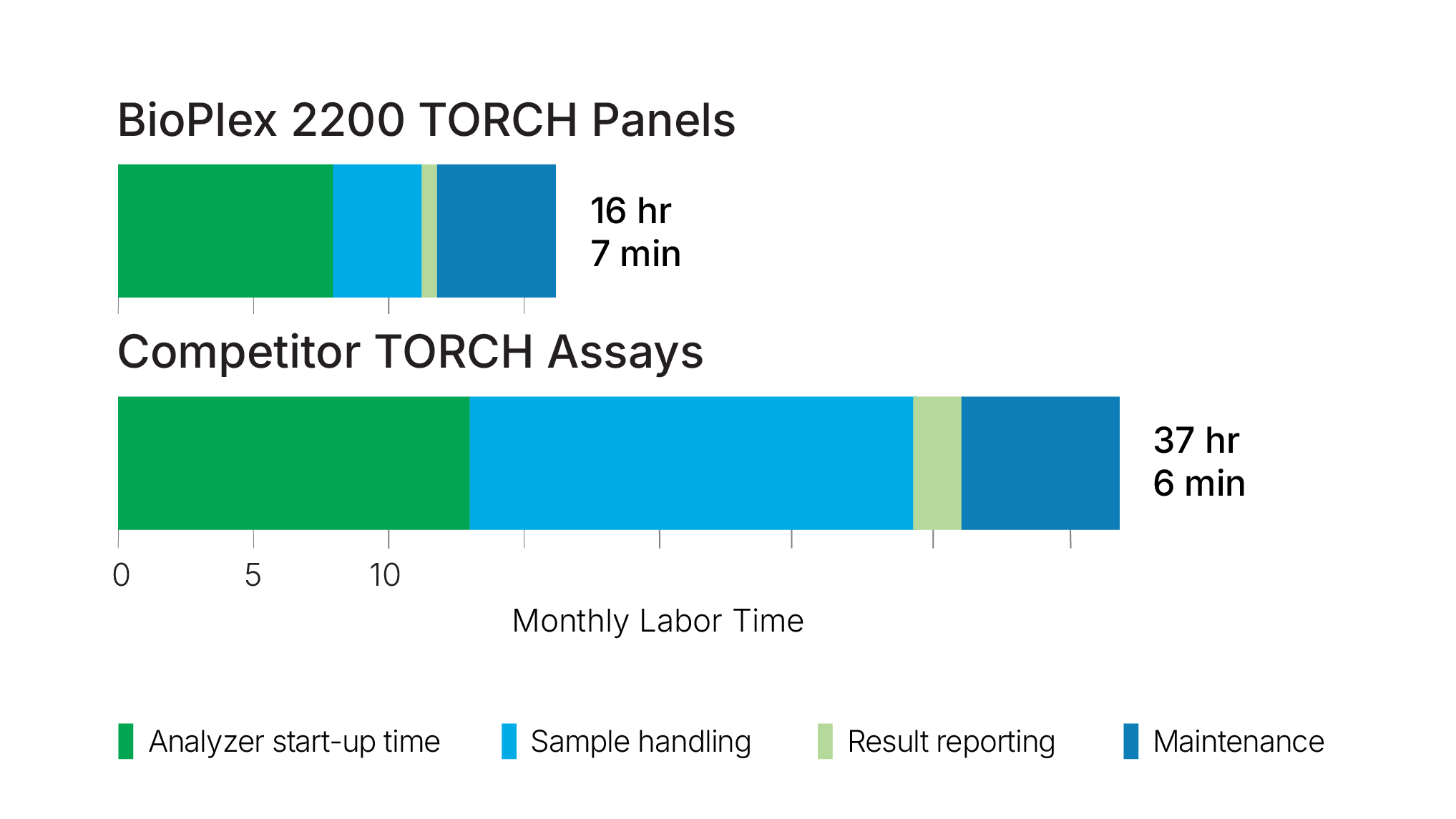
Case Study: Monthly Labor Time Comparison Table
See how you can reduce labor costs and make optimal use of medical laboratory technologists, your lab’s most valuable resource.
This case study compares the manual processing times required to perform TORCH testing with the BioPlex 2200 System versus a competitor’s fully automated system.
In total, using multiplexed TORCH testing required 57% less hands-on time compared to the competitor’s system.
For a personalized workflow analysis of your lab, please contact your Bio-Rad representatives.
More Results, Less Effort
The BioPlex 2200 TORCH panels leverage advanced multiplex testing to simplify and streamline your testing processes while ensuring uncompromised result quality.

Flexibility in Testing
Easily order multiple assays from a single test kit

Increase Laboratory Efficiency
Consolidate eight individual tests into three multiplex panels to simplify laboratory workflow and minimize effort

Report Results Confidently
Ensure high quality results with full automation and verification of assay performance in every test
Complementary Products

VIROTROL ToRCH Quality Control
An unassayed quality control for use with assays detecting IgG antibodies to Toxoplasma gondii, rubella virus, cytomegalovirus, and herpes simplex virus types 1 and 2.

VIROTROL ToRCH-M Quality Control
An unassayed quality control for use with assays detecting IgG and IgM antibodies to Toxoplasma gondii, rubella virus, and cytomegalovirus, and IgG antibodies to herpes simplex virus types 1 and 2.

VIROCLEAR ToRCH Quality Control
An unassayed, non-reactive quality control for use with assays detecting IgG and IgM antibodies to Toxoplasma gondii, rubella virus, cytomegalovirus, and herpes simplex virus types 1 and 2.

Liquichek ToRCH Plus Control
A bilevel, liquid control to monitor the performance of assays for primary ToRCH analytes and other widely used serology instruments and assays; includes a positive and a negative control.
Ordering
items
Use the filters below to refine results!









