The Western Blot Doctor is a self-help guide that enables you to troubleshoot your western blotting problems. In this section, you can find solutions to issues related to protein band appearance.
Other sections in the Western Blot Doctor:
- Band Appearance Problems
- Blot Background Problems
- Signal Strength Problems
- Band Size and Pattern Problems
Problems and Solutions
Click on the thumbnail that is most representative of your own blot to discover the probable causes and find specific solutions to the problem.
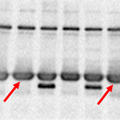
Problem: Inconsistent control protein levels among samples
|
Variation observed among the loading controls in each lane |
 |
| Possible causes: | Solutions: |
|
Samples may have different amounts of total protein |
|
|
Loading control protein levels may vary between test and control conditions |
|
Problem: Ghost protein bands
|
Nonspecific protein bands, can be large or out of place. |
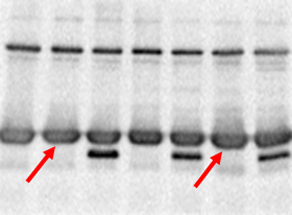 |
| Possible causes: | Solutions: |
| Sample overloading |
|
|
Too much antibody |
|
|
Exposure too long |
|
|
Rapid substrate consumption |
|
|
Blot was moved |
|
Problem: Swirls or missing bands; bands appear diffuse on blot
|
Bands do not look flat, may be trailing off in multiple directions. Bands may look broad and fuzzy. |
 |
| Possible causes: | Solutions: |
|
Contact between the membrane and the gel was poor; air bubbles or excess buffer remain between the blot and the gel (see also Blot Background > White spot) |
|
Problem: Blurry bands
|
(see also Protein Band Size and Pattern > Band(s) at slightly higher MW than expected) |
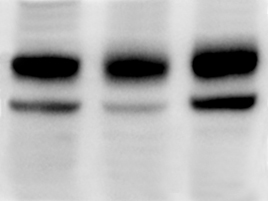 |
| Possible causes: | Solutions: |
|
Gel run at too high a voltage |
|
|
Trapped air bubbles present during transfer |
|
|
Incorrect running buffer composition |
|
Problem: Bands are curved (smiling) not straight
 not straight.jpg) |
|
| Possible causes: | Solutions: |
|
Running conditions were too fast, gel became overheated |
|
Problem: Broad or misshapen bands
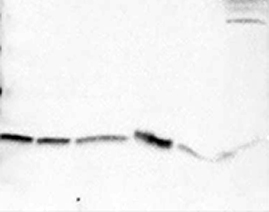 |
|
| Possible causes: | Solutions: |
|
Gel electrophoresis problems |
|
Problem: White (negative) bands on film using ECL method
|
|
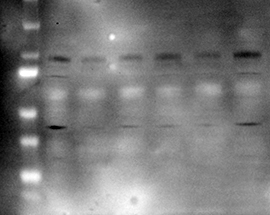 |
| Possible causes: | Solutions: |
|
Too much protein loaded |
|
|
Antibody concentration is too high |
|
Problem: No bands
| (see also Signal Strength Problems > Faint bands, weak or no signal) |  |
| Possible causes: | Solutions: |
|
Possible over-transfer or under-transfer |
|
|
Blocking agent interfering with signal |
|
|
Buffers may contain sodium azide, which inactivates HRP |
|
|
ECL detection reagents may be contaminated |
|
|
Peroxide may be inactive, resulting in lower peroxidase signal |
|
|
Inappropriate secondary antibody used |
|
|
Wrong concentration of antibody or low affinity to the target protein |
|
|
Antibody not suitable for western blotting |
|
|
Antibody activity loss due to long-term or improper storage |
|
|
Antigen not expressed in source material |
|
|
Not enough antigen loaded onto |
|