Welcome to the 2-D Doctor™ section on irregular spot patterns. The 2-D Doctor is a self-help guide that enables you to troubleshoot your 2-D gel issues. Here you will find solutions to the problem of irregular spot patterns on 2-D gels.
Other sections in the 2-D Doctor:
Problems and Solutions
Click on the thumbnail that is most representative of your own gel to find out the probable cause of and specific solutions to your problem.
For additional help, you can also view videos and browse FAQs.
Problem: Wavy Spots
Back to Top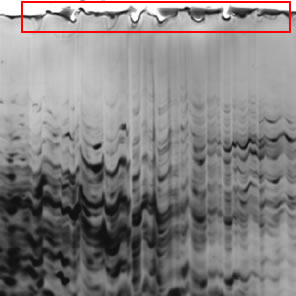
| Likely Cause | Insufficient overlay solution after gel casting |
| Recommended Solution | Overlay the gel with water-saturated butanol (n-butanol, l-butanol, or t-butanol) or t-amyl alcohol immediately after gel casting. These ensure that the gel has a clean, straight top edge. Use the overlay recommended by the manufacturer of the electrophoresis cell. |
| Recommended Product | Bio-Rad precast gels |
Problem: Local Wavy Disturbance of Spots
Back to Top
| Likely Cause(s) | SDS gel not evenly polymerized |
| SDS gel cassette leaking | |
| SDS gel cassette leaking during casting | |
| Recommended Solution(s) | Optimize the APS and TEMED concentrations. |
| De-gas solutions prior to the addition of APS/TEMED. | |
| Perform casting at room temperature, warming the glass plates if necessary. Be aware that the polymerization process is temperature dependent. If the temperature is too low, polymerization may be compromised. | |
| Recommended Product | Bio-Rad precast gels |
Problem: Missing High Molecular Weight Proteins
Back to Top
| Likely Cause(s) | Proteolysis |
| Low-quality or old reagents | |
| Large proteins not entering IPG strips during rehydration | |
| Large proteins not entering the second-dimension gels during electrophoresis | |
| Recommended Solution(s) | Include protease inhibitors in sample preparation buffers. |
| Use a combination of thiourea and urea in the sample preparation buffer. | |
| Use fresh reagents. | |
| Use a different rehydration method or rehydration buffer for better solubility. | |
| Increase equilibration time. | |
| Recommended Products | ReadyPrep™ total protein extraction kit |
| MicroRotofor™ cell lysis kits | |
| ReadyPrep 2-D starter kit equilibration buffer I | |
| ReadyPrep 2-D starter kit equilibration buffer II | |
| Bio-Rad iodoacetamide |
Problem: Vertically Doubled Spots
Back to Top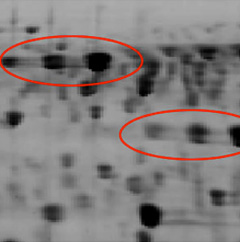
| Likely Cause(s) | IPG strip improperly placed on SDS gel |
| Temperature gradient occurring in the gel during the second-dimension gel run | |
| Low-quality or old reagents | |
| Improper equilibration | |
| Polypeptides not sufficiently reduced | |
| Recommended Solution(s) | Ensure that the IPG strip is in direct contact with the second-dimension gel along its entire length. To prevent twisting of the IPG strip during placement, press the plastic backing of the IPG strip firmly against one of the 2-D gel plates. Use staggered plates, as they allow easier placement of the IPG strip. |
| Reduce the maximum current. Refer to the instruction manual for the electrophoresis cell for recommended running conditions. | |
| Equilibrate the IPG strips with the recommended volume of buffer for the recommended amount of time, or longer if necessary. | |
| Use fresh reagents, including Iodoacetamide and DTT. | |
| Use a better circulation system for even heat dissipation in the electrophoresis cell during a run. | |
| Recommended Products | Bio-Rad precast gels |
| Bio-Rad glass plates | |
| Bio-Rad gel casting chambers | |
| Bio-Rad iodoacetamide | |
| Bio-Rad dithiothreitol, 1g | |
| Bio-Rad dithiothreitol, 5g |
Problem: Diffuse and Uneven Background in Silver-Stained Gel
Back to Top
| Likely Cause(s) | Insufficient washing steps |
| Insufficient fixative steps | |
| Contaminant in agarose overlay solution or running buffer | |
| Recommended Solution(s) | Ensure that the gels are fully immersed and floating properly in the staining solution. They should not stick to the staining tray. Do not place too many gels in one tray. |
| Perform more washing steps if needed. Ensure that the water used is of high quality, and that the staining tray is clean. | |
| Some uneven background stain is normal when using a silver stain. Due to the different chemicals and ions migrating into the gels, some regions can be stained with different colors or intensities. Often, a longer fixing procedure can minimize this effect. | |
| If you are still experiencing difficulty, use a different stain, such as Flamingo™ fluorescent gel stain or Oriole™ fluorescent gel stain. | |
| Recommended Products | Dodeca™ stainer |
| Dodeca silver stain kit | |
| Silver Stain Plus™ kit | |
| Flamingo fluorescent gel stain | |
| Oriole fluorescent gel stain |
Problem: Localized Diffuse & Uneven Background in Silver-Stained Gel

| Likely Cause(s) | Insufficient washing steps |
| Insufficient fixative steps | |
| Contaminant in agarose overlay solution or running buffer | |
| Recommended Solution(s) | Ensure that the gels are fully immersed and floating properly in the staining solution. They should not stick to the staining tray. Do not place too many gels in one tray. |
| Perform more washing steps if needed. Ensure that the water used is of high quality, and that the staining tray is clean. | |
| Some uneven background stain is normal when using a silver stain. Due to the different chemicals and ions migrating into the gels, some regions can be stained with different colors or intensities. Often, a longer fixing procedure can minimize this effect. | |
| If you are still experiencing difficulty, use a different stain, such as Flamingo fluorescent gel stain or Oriole fluorescent gel stain. | |
| Recommended Products | Dodeca stainer |
| Dodeca silver stain kit | |
| Silver Stain Plus kit | |
| Flamingo fluorescent gel stain | |
| Oriole fluorescent gel stain |
Page Contents
Videos
Documents
TEST
Number Description Options
| Number | Description | Options |
|---|---|---|
| 6222 | IPG Equilibration for the Second Dimension, Placement and Agarose Embedding of IPG Strips | Click to download |
| 6236 | Using Precison Plus Protein Standard Plugs | Click to download |







