To achieve straight runs and good resolution in pulse field gel electrophoresis (PFGE) it is necessary to create homogeneous electrical fields. There have been multiple approaches to PFGE but the combination of clamped homogeneous electric fields (CHEF), pressure assisted capillary electrophoresis (PACE), and dynamic regulation (DR) technologies works best in creating the homogeneous electrical fields that ensure consistency and run-to-run reproducibility. This section provides an overview of PFGE and the research areas where PFGE can be used.
Page Contents
CHEF technology is the leading PFGE technique and resolves DNA over a wide range of molecular weights in a straight lane (Chu et al. 1988). It employs the principles of contour-clamped electrophoresis to generate homogeneous electrical fields by holding the electrodes at intermediate potentials, thus generating the homogeneous electric fields necessary for straight, distortion-free lanes. This instrumentation allows the manipulation of pulse time, field strength, and pulse angle, all of which influence the migration rate of DNA through an agarose gel and the resolution of the separation.
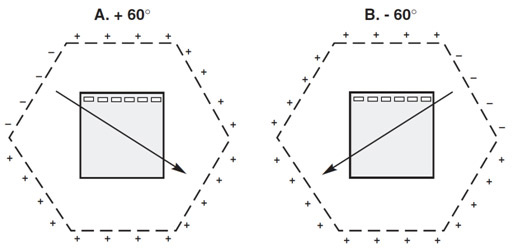
Voltage clamping by the CHEF Mapper® system. A, Relative electrode potentials when the +60° field angle is activated; B, Relative electrode potentials when the –60° field angle is activated.
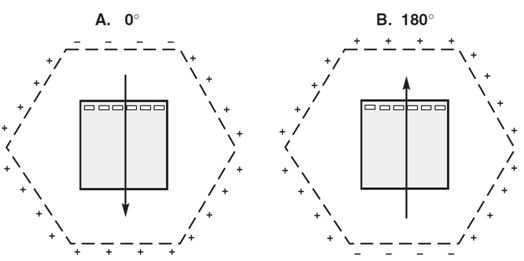
Voltage clamping by the CHEF Mapper® system in the FIGE mode. A, Relative electrode potentials when the 0° field vector is activated; B, Relative electrode potentials when the 180° field vector is activated.
Pulse time is the period of the alternating field to which DNA molecules are subjected in PFGE. Resolution will be optimal when the re-orientation time for the DNA molecules is comparable to the pulse time. The DNA migration rate increases as the field strength or voltage increases. However, greater migration is accompanied by decreased band sharpness. In selecting the field strength for an experiment, a compromise between run time and resolution has to be made. Field angle affects separation as well, with the mobility of large DNAs (>1 Mb) increasing as the field angle is decreased. This is accompanied by decreased resolution of smaller DNAs. Software is available to aid in the optimization of all three of these parameters to suit specific DNA size ranges.
Programmable autonomously controlled electrodes (PACE; Clark et al. 1988) technology allows users to select the angle of electrophoretic pulsing optimal for the desired size range by independently controlling each electrode. This results in the simulation of virtually any pulsed field technique.
Dynamic regulation (DR) is the electronics design by which each of the 24 electrodes is regulated; CHEF-DR® systems are capable of compensating for changes in buffer conductivity or gel size, thereby preventing these changes from affecting the reproducibility of results.
Field inversion gel electrophoresis (FIGE) is a simple variant of PFGE used for rapid sample resolution in the 100 bp–250 kb size range in which the orientation of the electric field is switched by 180°. The polarity of the field is simply reversed periodically along the axis of migration. The duration of the pulses is unequal so that there is a net migration in a single direction. FIGE only allows separation up to 750 kb, but one of its advantages is that it can be performed with conventional agarose gel electrophoresis equipment. The only additional apparatus required is a pulse controller to periodically change the polarity of the field provided by the power supply;
Asymmetric field inversion gel electrophoresis (AFIGE) is a further refinement of the FIGE technology; AFIGE applies a different voltage to the forward direction electrical field but not to the reverse direction electrical field, thus optimizing the sample resolution in the FIGE size range.
Many pulsed field gel electrophoresis techniques, based on periodically changing the orientation of the electric field, have been developed. This section provides a brief overview of these alternative electrophoresis techniques.
Orthogonal Field Alternating Gel Electrophoresis (OFAGE)
OFAGE uses two sets of electrodes. It establishes the separation of large DNA molecules but does not result in straight migration lanes because the electrical fields are not homogeneous. This technique was therefore abandoned when it became clear that high resolution separation was possible using homogeneous fields.
Transverse Alternating Field Electrophoresis (TAFE)
TAFE is performed by orienting the electric fields transverse to the gel (Gardiner et al. 1986). The electrodes are placed at either side of the gel, which is mounted in a vertical orientation. The lanes are straight but the resolution is limited because the angle of electrophoresis is changing as the DNA migrates down the gel. As a result, the bands are compressed.
Rotating Gel Electrophoresis (RGE)
RGE is a technique in which the electric field is reoriented by physically moving the gel with respect to fixed electrodes (Serwer 1987). This technique has been largely superseded by techniques in which the gel remains stationary and the field is manipulated by multiple independently controlled electrodes.
Carle GR and Olson MV (1984). Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res 12, 5647–5664.
Chu G et al. (1986). Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science 234, 1582–1585.
Clark DM et al. (1988). A novel instrument for separating large DNA molecules with pulsed homogenous electric fields. Science 241, 1203–1205.
Gardiner K et al. (1986). Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somatic Cell Mol Genet 12, 185–195.
Serwer P (1987). Gel electrophoresis with discontinuous rotation of the gel: An alternative to gel electrophoresis with changing direction of the electrical field. Electrophoresis 8, 301–304.

