Develop Safe & Effective Cell and Gene Therapies
Use Vericheck ddPCR™ Contaminant Testing Solutions to Precisely Detect Impurities
Impurities in Product & Processes
Droplet Digital PCR Applications for Impurity Testing — Providing Reproducible, Accurate, and Sensitive Results for Contaminants
Cell and gene therapies have rapidly gained popularity but ensuring safety and effectiveness in patients remains complex with no room for error. Research, development, and manufacturing laboratories around the world are leveraging the precision and convenience of Bio-Rad's Droplet Digital™ PCR (ddPCR™) Contaminant Testing Solutions to address critical challenges for a range of applications in cell and gene therapy to meet stringent regulatory requirements and improve product safety.
The FDA requires a low coefficient of variation (CV) in bioanalytical measurements, with overall accuracy and precision at each concentration level within ±15% of the nominal concentration. Existing qPCR-based QC methods are reliant on standards that are difficult to source and can be variable from batch-to-batch, adding time and cost. In addition, qPCR-based methods are sensitive to PCR inefficiencies caused by secondary structure or in process inhibitors leading to assay failures and expensive assay repeats. These problems also are reflected in the challenges transferring from process development to either internal manufacturing groups or externally to a contract development manufacturing organization (CDMO). To assist with these challenges, Bio-Rad has developed solutions utilizing ddPCR technology to make impurities testing easier and more reliable.
Gene Therapy
ddPCR Applications for Gene Therapy
Detecting potentially harmful contaminants left behind by gene therapy manufacturing processes is essential to ensure patient safety and product efficacy for product release. However, several challenges complicate quality control (QC) testing, including accurate viral titer measurement, precise characterization of empty-full capsid ratios, rapid detection of mycoplasma and replication-competent viruses, and ensuring vector purity.
Traditional testing methods often require high sample amount, have lengthy turnaround times and low inhibitor tolerance, or are prone to variability increasing production costs. Additionally, finding sensitive methods offering all-in-one quantification and detection of host cell DNA and pathogenic microbes in gene therapy batches can be challenging. Without methods that can quickly deliver reliable results, you may end up increasing production costs by continuing to produce contaminated lots or, in the case of false positives, you might unnecessarily delay production when a lot is within specifications.
Fortunately, Bio-Rad’s ddPCR™ Contaminant Testing Solutions offer ready-to-use and custom assays for impurity testing that are designed to overcome these QC challenges and expedite your gene therapy manufacturing processes.
| In-Process Testing | In-Process Testing | Patient Monitoring | ||||
|---|---|---|---|---|---|---|
| Transfection | Virus Purification | Virus Production and Recovery | Potency | Purity | Safety | Verify Therapeutic Delivery and Expression |
| Plasmid Quality | Viral Titer, Capsid Titer, Empty-Full Ratio | Plasmid Quality | Mycoplasma | Gene Expression | ||
Cell Therapy
ddPCR Applications for Cell Therapy
For gene-modified cell therapies like CAR-T cells, ddPCR technology offers absolute quantification of transgene copy numbers in a sample. This method can be used to provide an accurate measurement of CAR copy numbers in transduced T cells or count the number of cells that contain the transgene of interest — an invaluable feature for transgenes undetectable by antibodies.
With Droplet Digital™ PCR (ddPCR™) technology, you can have it all — easy-to-use plate-based instruments that deliver quantitative accuracy, sensitivity, and reproducibility.
| In-Process Testing | Final QA/QC | Patient Monitoring | ||||
|---|---|---|---|---|---|---|
| Incoming Vector | Activate and Transduce T Cells | Prepare Final Cell Dose | Potency | Purity | Safety | Verify Therapeutic Delivery and Expression |
| Plasmid Quality | Transgene Expression | Vector Copy Number | Residual DNA | Mycoplasma | Gene Expression | |
Vericheck ddPCR Contaminant Testing Solutions
“Truth in Testing”
In cell culture and during the manufacturing of cell-based vaccines, biologics, and gene therapies, monitoring a product’s critical quality attributes and producing contaminant-free batches is critical for patient safety. Accurate characterization of empty to full capsids presents an additional challenge, as high capsid content variability can hinder therapeutic potency and safety. ddPCR™ provides highly precise and absolute quantification of nucleic acids by counting nucleic acid molecules encapsulated in discrete water-in-oil droplets. Digital measurement of nucleic acids using ddPCR technology is suitable for analysis of both in-process samples and final drug products due to the technology's tolerance to PCR inhibitors. The tolerance to formulation matrices enables the implementation of ddPCR technology throughout the entire manufacturing process with multi-sample compatibility, low-sample input, and fast turnaround time. Bio-Rad's ddPCR systems utilize a simple workflow with minimal hands-on time, while reducing sample replicates and user-to-user variability.
Empty-Full Capsid Ratio

Adeno-associated virus (AAV) has become the preferred vector for gene therapy and assessing the ratio of empty to full capsids is a critical and required step in AAV production. Previously, a significant amount of precious sample was required. Now, with the absolute quantification of Droplet Digital™ PCR, the Vericheck ddPCR Empty-Full Capsid Kit requires only small sample amount of crude lysate or purified samples, which is ideal for any step in the AAV vector development or manufacturing process.
Vericheck ddPCR Empty-Full Capsid Kit Benefits
- Quantify empty-full ratio and viral titer in one assay
- Analyze crude or purified samples
- Reduce sample input requirements
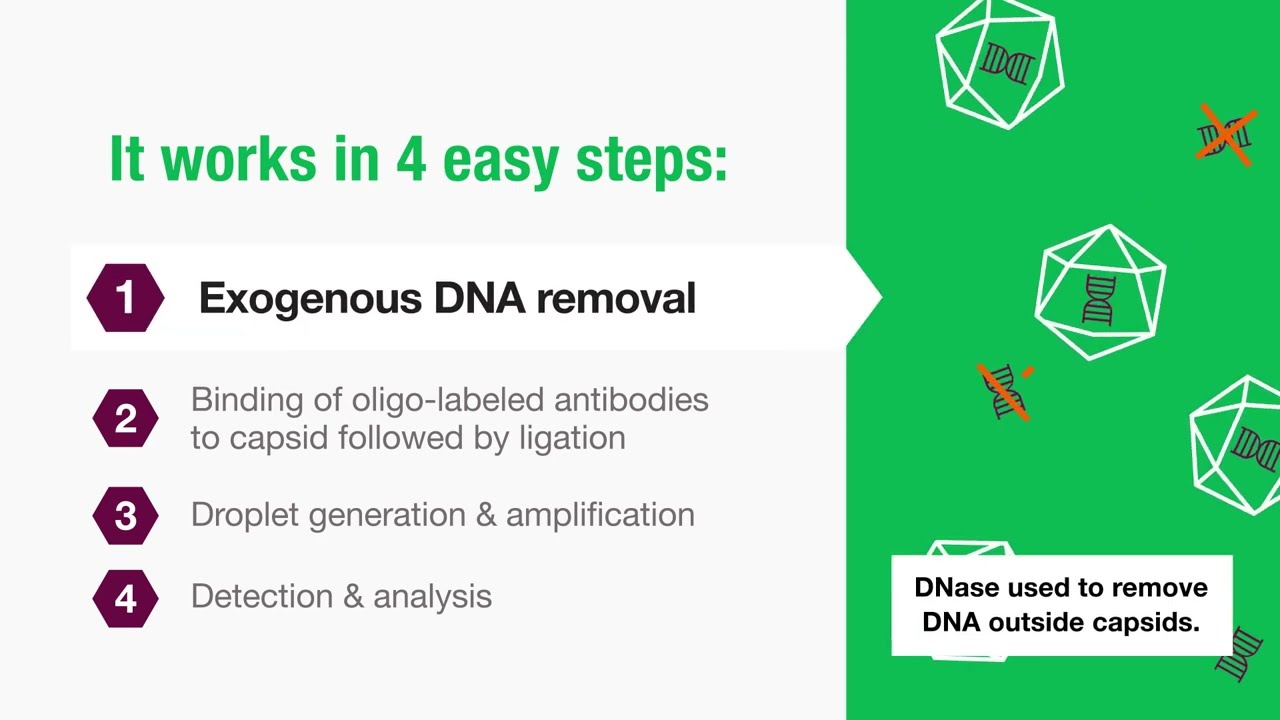
How it Works: Vericheck ddPCR Empty-Full Capsid Kit
Determining the AAV empty/full capsid ratio is a key quality attribute, but current methods are imprecise, expensive, and time-intensive. By combining the measurement of capsid proteins and viral titer with the high precision of Droplet Digital PCR (ddPCR), the Vericheck ddPCR Empty-Full Capsid Kit offers reliable, accurate, and comprehensive data with a single assay for samples ranging from crude to purified.
Mycoplasma Testing
Cell lines and patient samples are prone to outbreaks of various Mycoplasma infections; therefore, it is essential to address outbreaks as soon as they occur through robust testing to prevent product and time loss. The Vericheck ddPCR Mycoplasma Detection Kit is a probe-based, highly sensitive and highly specific Mycoplasma detection solution. This kit is designed and validated to meet European, U.S. and Japanese pharmacopoeia requirements. Detect up to 112 Mycoplasma species using Droplet Digital. Utilize the premium edition software with audit trails and tools for U.S. FDA 21 CFR Part 11 compliance to easily analyze and report your data.
Vericheck ddPCR Mycoplasma Solution Benefits
- Verify Mycoplasma presence/absence with absolute quantification
- Reduce variability, plate-to-plate and user-to-user, giving highly reproducible results
- Lower cross reactivity with related species, providing low false positive results

Did You Know That Bio-Rad Can Help You Choose a Mycoplasma Detection Strategy?
Mycoplasma contamination in cell cultures is a prevalent and expensive issue in biopharma, making it essential to choose an effective detection strategy. Watch the video to discover how ddPCR™ technology can help you save time and reduce the risk of false positives. Ensure reliable testing and maintain smooth lab operations with the Vericheck ddPCR Mycoplasma Detection Kit.
Residual DNA
Residual DNA content is a common impurity in cell and gene therapy manufacturing, and levels must be carefully monitored for lot release. Droplet Digital PCR is a precise and sensitive method for direct quantification of residual DNA content. Since Droplet Digital PCR provides multi-sample type compatibility and does not rely on reaction efficiency for quantification, it is less prone to sample interference from complex matrices and process intermediates thus reducing sample preparation time. Droplet Digital PCR provides a highly sensitive method for direct quantification of residual HCD to ensure adherence to the FDA guideline of <10 ng/dose.
Human gene therapy products are produced in immortalized cell lines, most commonly HEK293. Residual DNA from immortalized cell lines can harbor tumorigenic genetic sequences or retroviral sequences posing significant risks to patients. The FDA recommends that manufacturers degrade any residual DNA to a size below that of a functional gene, defined as <200 base pairs. Manufacturers require an inexpensive, accurate, and reliable way of measuring the size of residual DNA, which ddPCR technology can provide. The Vericheck ddPCR HEK293 Sizing Kit is the first HEK293 specific ddPCR based sizing solution that has been designed, and validated to meet regulatory guidance requirements and capable of measuring HEK293 size without the use of reference curve.

Vericheck ddPCR HEK293 Residual DNA Detection Kit
The Vericheck ddPCR HEK293 Residual DNA Quantification Kit brings the specificity in testing for HEK293 DNA contamination. The precision and reproducibility of this kit allows for an absolute measurement of residual HEK293 DNA using Droplet Digital PCR.

Vericheck ddPCR HEK293 Sizing Kit
The Vericheck ddPCR HEK293 sizing kit is the first HEK293 specific ddPCR based sizing solution, designed, and validated to meet regulatory guidance requirements and capable of measuring HEK293 size without the use of reference curves.

Vericheck ddPCR E. coli Residual DNA Quantification Kit
Use the ddPCR E. coli Residual DNA Quantification Kit to detect femtogram amounts of residual E. coli host cell DNA (HCD) in various types of bioproduction intermediates.

Vericheck ddPCR CHO Residual DNA Detection Kit
Use the ddPCR CHO Residual DNA Quantification Kit to detect femtogram amounts of residual CHO host cell DNA (HCD) in various types of bioproduction intermediates.
Replication Competent Virus Testing
Vericheck ddPCR Replication Competent Virus Testing Kits
Replication Competent Viruses (RCVs) are virus particles that can infect cells and replicate to produce infectious viral particles. RCV testing of samples are carried out during different stages of the viral production workflow. The Vericheck ddPCR RCL (Lentivirus) and RCAAV (AAV) testing kits have been designed to run in-house ddPCR based assays for confirming the presence or absence of replication competent Lentivirus or Adeno-Associated viruses during the production of cell and gene therapy drug products.

Droplet Digital PCR Solutions
Bio-Rad's ddPCR systems eliminate the need for a standard curve, simplifying QC workflows with minimal hands-on time while reducing sample replicates and user-to-user variability. ddPCR technology is also more resistant to in process matrix components that inhibit traditional qPCR efficiency.