Selecting an Anion Exchange Column
The choice of an anion exchange column depends on a number of factors affecting chromatographic separation, including the charge characteristics of both target molecule(s) and other molecules in the sample, the volume and concentration of the sample, and the flow rate and pressure limits of the chromatography system. Bio-Rad offers an extensive range of anion exchange columns to cover your needs. The different types of anion exchange columns have been optimized for a number of applications:
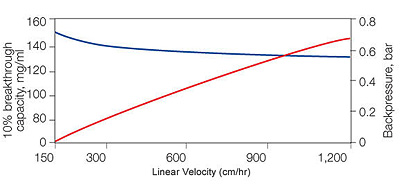
Binding and backpressure properties of UNOsphere Q support:
A 5 mg/ml sample of BSA in 10 mMTris, pH8.5, was loaded onto a 1.1 x 20 cm column. Red, backpressure; blue, 10% breakthrough capacity.
- High-resolution separations
- Applications from preparative separations to polishing
- Running at low, medium, or high flow rates
- Covering different analyte size ranges
- Compatibility with commonly used buffer systems and salts
- Matrices with either strong or weak functional groups
- Different matrix types including macroporous and continuous nonporous
- Low backpressure
- Compatibility with different sample viscosities and operating temperatures
Anion Exchange Column Design
Ion exchange chromatography is used to separate charged molecules. In an anion exchange column, the packing is positively charged and therefore retains negatively charged molecules by coulombic interaction. The bound molecules are eluted with an anion gradient. For molecules with multiple charged groups, such as proteins, it is the overall charge in a given buffer chemistry (pH, etc.) that determines whether and how strongly the molecule will bind to the column stationary phase.
Anion exchange chromatography columns are available with matrices having either strong or weak functional groups. The strong anion exchange matrix is quaternary ammonium, and is designated Q. The weak anion exchange matrix is diethylaminoethyl, or DEAE. An intermediate strength resin, which contains primarily tertiary with some quaternary amines, is also available.
The pH of the buffer in the column determines the charge of each of the constituents in a sample. The charge of strong anion exchange resins is minimally altered by a change in pH and can operate over a broad pH range. DEAE is more affected by changes in pH, with a usual operating range of pH 7–10.
The availability of a wider working pH range with strong anion exchange columns provides the ability to use pH to change the net charge of the target molecules to make them bind more strongly, more weakly, or not at all. Chromatography may be carried out in positive mode, where the target(s) of interest bind strongly to the column and (many) other molecules do not bind as strongly. Negative mode is common in polishing, where contaminants bind to the anion exchange column and the target molecule does not bind and is thus not retained on the column.
Elution is usually achieved with a salt gradient. The most common ions are PO43–, SO42–, COO–, and Cl–. Choice of anion depends on the desired elution profile and which ions are compatible with future purification or future use of the analyte(s).
Applications of Anion Exchange Columns
Anion exchange columns are used extensively for the purification of biomolecules. There is a wide selection of column types, sizes, and different strengths of functional groups. Combined with optimized binding and elution conditions, anion exchange column chromatography can be used at all stages of biomolecule isolation and analysis including initial cleanup, purification, separation of analytes, and removal of contaminants such as endotoxins, host cell proteins, or ionic compounds.
Anion Exchange Chromatography Systems, Accessories, and Media
-
Chromatography Systems
-
Chromatography Cables, Fittings, and Tubing
-
Anion Exchange Chromatography Media
Related Topics
Cation Exchange Column, HPLC Column, High Performance Liquid Chromatography, Gel Filtration Column, Gel Filtration Chromatography, Chromatography Systems, Biotechnology Equipment