
- Life Science
- Introduction to Chromatography
- Introduction to Affinity Chromatography
- Introduction to Gravity Chromatography
- Introduction to Hydrophobic Interaction Chromatography
- Introduction to Ion Exchange Chromatography
- Introduction to Low-Pressure Chromatography Systems
- Introduction to Medium Pressure Chromatography Systems
- Introduction to Multimodal or Mixed-Mode Chromatography
- Introduction to Size Exclusion Chromatography
- Types of Chromatography
Chromatography is a family of related techniques for the separation of different molecules or components within a mixture using phase equilibrium partitioning. This section describes the basic types of chromatography and their applications, their general configurations of various chromatography systems, the most common types of chromatography media and the underlying chemistry, and how to choose the right media for your application, focusing on standard protein purification methods.
Related Topics: Liquid Chromatography Principles, Column Chromatography Methods and Instrumentation
Page Contents
Chromatography enables the separation of compounds in a mixture by dissolving the mixture in a mobile phase and passing this mixture over a stationary phase. Molecules that interact strongly with the stationary phase (or have a greater affinity for the stationary phase than the mobile phase) migrate through the resin slowly, whereas molecules that interact weakly with the resin move through it quickly. The purpose of this separation can be analytical or preparative. The goal of analytical chromatography is to identify, and in some cases, quantify, components of complex mixtures. Preparative chromatography, in contrast, aims to isolate or purify molecules of interest for downstream uses.
The physical setup of a chromatography system and the compositions of the mobile and stationary phases depend on the characteristics of the compounds to be separated. The physicochemical parameters must be tuned so that the molecules of interest move through the stationary phase at different speeds as the mobile phase flows through it, resulting in a resolution of the original mixture into several fractions. In some cases, the fractionation is of a binary nature: compounds of interest are either retained by the stationary phase or pass through it; in other cases, chromatography results in a continuous stream of discrete fractions. Follow the links at the end of each section below to learn more about different chromatography methods and systems.
Preparative chromatography refers to the isolation or purification of target molecules. A common application of preparative chromatography is laboratory-scale protein purification for biochemical characterization; preparative chromatography is also used in the biopharmaceutical industry for process-scale protein purification.
In contrast, analytical chromatography uses the separation of molecules to identify and quantify components of mixtures. Among other applications, analytical chromatography can be used to follow the fates of substrates in chemical reactions, to test for the presence of substances of interest in complex mixtures, and to profile metabolic activities.
There are two main types of chromatography, gas chromatography (GC) and liquid chromatography (LC). Either may be used for analytical or preparative work, and both are usually performed by passing the mobile phase through a column containing the stationary phase, although liquid chromatography can use other configurations.
Gas chromatography separates molecules based on their boiling points and their interaction with the stationary phase. Mixtures to be separated are heated past the boiling of their least volatile component. They are then swept across the solid phase by an inert gas, hence the name gas chromatography. The stationary phase in gas chromatography is the inside surface of a long capillary column coated with a liquid or polymer through which the gas mixture flows. Although GC can be used for preparative purposes, it is most commonly used for analytical work.
Liquid chromatography separates compounds dissolved in a liquid mobile phase by passing the liquid over a solid stationary phase, the chromatography media, or resin, with which the compounds have differing degrees of interaction. Individual components of the mobile phase are thus more or less retarded in their passage through the chromatography media. LC is further subdivided into planar chromatography and column chromatography.
When liquid chromatography is carried out in a single plane it is referred to as planar liquid chromatography; here, the liquid mobile phase passes through a solid stationary phase such as a strip of paper (paper chromatography) or silica gel that is immobilized on a glass slide (thin-layer chromatography/TLC).
Thin-layer chromatography (TLC) is a common form of planar chromatography. TLC is a qualitative analytical chromatography method used to separate nonvolatile molecules. TLC is an improvement over the original form of planar chromatography using blotter paper because the properties of the stationary phase and solvent transport can be more tightly controlled.
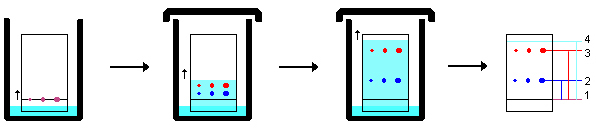
Fig. 1. Thin-layer chromatography. Analyte mixture (pink spots) is spotted at the bottom of a TLC plate and the plate is placed into a solvent (cyan). The solvent travels up the TLC plate via capillary action. Analytes travel with the mobile phase and separate (red vs. blue spots) based on their interaction with the stationary and mobile phase. Analytes can then be identified based on the distance traveled in a given solvent and period of time.
For TLC, the mixture to be analyzed is spotted on the bottom of a glass plate that is coated with a thin layer of stationary phase, commonly a silica gel. The bottom of the plate is then placed into a jar or tank with a small amount of solvent at the bottom, wetting the stationary phase. As the solvent travels up the TLC plate via capillary action, it acts as the mobile phase, carrying the compounds to be analyzed with it. Separation of analytes is achieved because different analytes interact with the mobile and stationary phase to different extents, and thus travel up the TLC plate at different rates. For a given solvent and stationary phase, each compound will have a characteristic retention factor (Rf) that can be used to identify it. By applying standards of known compounds in tightly controlled spots, for example using automated instrumental application, TLC can yield semiquantitative results. This method is referred to as high-performance TLC (HPTLC). TLC is typically used for rapid monitoring of the progression of chemical reactions or inexpensive, low-tech analysis of simple or crude mixtures.
Visit the Liquid Chromatography Principles page for a description of the methods used for liquid chromatography.
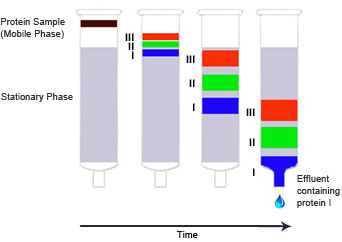
Fig. 2. Column chromatography. Protein I has a weak affinity for the stationary phase and passes through the column rapidly. Protein III has high affinity for the stationary phase and moves through the column slowly; it has a high retention time. These two proteins can thus be separated based on their different retention times.
Column chromatography typically refers to liquid chromatography performed on a three-dimensional stationary phase packed inside of a glass, plastic, or metal column and can be used for both preparative and analytical purposes. Based on the characteristics of the compounds to be separated and the compositions of the mobile and stationary phases, different compounds will move through the column at different speeds as the mobile phase flows through it, enabling the collection of discrete fractions at the outlet containing one or more components of the original mixture.
Column chromatography techniques encompass a broad range of applications based on diverse solid and liquid phase chemistries and column configurations, enabling precise analyses and the resolution of complex mixtures that would be difficult or impossible to separate by other means.
Column chromatography can be performed using gravity flow or with the aid of various types of pumps and specialized instrumentation to control the flow of the fluid phase through the column. Visit the Column Chromatography page for an overview of the different types of systems used for column chromatography.
Chromatography stationary phases, also known as chromatography media or resins, vary widely in composition. One or more media types may be used depending on the properties of the molecules of interest to be separated. Molecules can be separated based on the following physical properties (each using different methods):
- Charge (ion exchange chromatography/IEX)
- Hydrophobicity (hydrophobic interaction chromatography/HIC)
- Size (size exclusion chromatography/SEC)
- Affinity (affinity chromatography)
- Multiple properties (multimodal or mixed-mode chromatography)
- A combination of the above
Some chromatography media are applicable to a broad range of separations, whereas other media are designed for a limited application to the separation of a unique class of molecules, for example, antibodies. Follow the links above to learn more about each type of chromatography method, or visit the Types of Chromatography page for an overview.
