
Fig. 1. The ChemiDoc MP Imaging System and fluorescent western blotting workflow. A complete family of instruments, reagents, and antibodies to obtain fluorescent western blot results reliably, reproducibly, and with high data quality.
HERCULES, Calif. — February 2, 2017 — Bio-Rad Laboratories Inc. (NYSE: BIO and BIOb) today announced the launch of the ChemiDoc MP Imaging System. The imager combines the industry’s best performance with ease of use and is ideal for researchers who need reliable, reproducible fluorescent western blotting data. The system offers high-resolution DNA and protein gel documentation features and its chemiluminescence detection sensitivity matches that of traditional X-ray film. The ChemiDoc MP System is one platform solution that meets all researcher needs for blot and gel imaging.
More proteins can be measured on a single blot
Fluorescent imaging enables researchers to quantify multiple proteins in a single measurement, an advantage over chemiluminescent western blots that require stripping and reprobing or cutting membranes to measure different proteins. Also advantageous is obtaining more data from less sample, especially when researchers want to study different isoforms of proteins or evaluate expression and interaction in complex pathways.
With the ChemiDoc MP Imaging System, researchers can detect a larger portion of the light spectrum than ever before. The system, which has five fluorescent channels, can detect both visible light (RGB) and near infrared (NIR) fluorescence, allowing simultaneous detection of up to three proteins on a single blot. Traditional fluorescent scanners, in contrast, are limited to detecting only NIR signal and up to two proteins simultaneously.

Fig. 2. Triplex western blot imaged by the ChemiDoc MP Imaging System:
Target protein #1 (ATG7): Red — StarBright™ Blue 700 Secondary Antibodies*
Target protein #2 (AKR1C2): Green — DyLight 800
Normalization protein (tubulin): Blue — hFAB™ Rhodamine Antibodies*
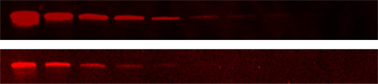
Fig. 3. Detection of AKR1C2 in a twofold dilution series of whole cell lysate. ChemiDoc MP (top), compared to a competitive platform (bottom).
* Fluorescent-labeled antibodies exclusive to Bio-Rad Laboratories.
ChemiDoc MP Imaging System is the most sensitive system available for both fluorescent and chemiluminescent detection. In a side-by-side comparison of the ChemiDoc MP Imaging System and a traditional fluorescent laser scanning system, the ChemiDoc MP Imaging System provided greater sensitivity (Figure 3).
Superior normalization options for better data reliability
The ChemiDoc MP Imaging System gives researchers the best results available when performing stain-free total protein normalization (TPN), a method referenced in more than 400 peer-reviewed papers since its 2010 introduction. TPN, endorsed by the Journal of Biological Chemistry as the preferred method for normalizing protein expression levels, normally requires a staining step that can be time-consuming and error prone. Using the ChemiDoc MP Imaging System when performing stain-free TPN eliminates this step by using a polyacrylamide gel chemistry to make proteins fluoresce directly in the gel.
For scientists who want to continue using their validated housekeeping proteins for protein normalization, Bio-Rad offers human fragment antigen-binding primary antibodies labeled with rhodamine for use as common housekeeping proteins. Bio-Rad generates these using HuCAL® technology and they can be used with other primary/secondary antibodies from different species without cross-reactivity. These labeled primary antibodies offer a method for one-step detection of housekeeping proteins — without the need for a secondary antibody — that is more reliable and easier to use than traditional anti-housekeeping antibodies.
Easy to use with well-known Bio-Rad support
Like other Bio-Rad instruments, the ChemiDoc MP Imaging System is intuitive and user-friendly. Features include application-driven selection of optimal light sources and filters along with help overlay screens that allow researchers to tap, explore, and learn in minutes.
For researchers new to western blot imaging systems, Bio-Rad continues to lead the industry in customer support with online resources, including Western Blot Doctor™ as well as a library of videos.
Learn more about Bio-Rad’s complete line of western blotting products here.
DyLight is a trademark of Thermo Fisher Scientific Inc.
About Bio-Rad
Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb) develops, manufactures, and markets a broad range of innovative products and solutions for the life science research and clinical diagnostic markets. The company is renowned for its commitment to quality and customer service among university and research institutions, hospitals, public health and commercial laboratories, as well as the biotechnology, pharmaceutical, and food safety industries. Founded in 1952, Bio-Rad is based in Hercules, California, and serves more than 100,000 research and healthcare industry customers through its global network of operations. The company employs over 8,000 people worldwide and had revenues exceeding $2 billion in 2015. For more information, please visit www.bio-rad.com.
This release may be deemed to contain certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements we make regarding plans to introduce products to the molecular diagnostics testing market and our development and launch of new products. Forward-looking statements generally can be identified by the use of forward-looking terminology such as “plan”, “believe,” “expect,” “anticipate,” “may,” “will,” “intend,” “estimate,” “continue,” or similar expressions or the negative of those terms or expressions, although not all forward-looking statements contain these words. Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. These risks and uncertainties include product quality and liability issues, our ability to develop and market new or improved products, our ability to compete effectively, and international legal and regulatory risks. For further information regarding our risks and uncertainties, please refer to the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operation” in Bio-Rad’s public reports filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K and our Quarterly Report on Form 10-Q. Bio-Rad cautions you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. We disclaim any obligation to update these forward-looking statements.
For more information contact:
Bio-Rad Laboratories, Inc.
Raymond R. Miller
510-680-0674
raymond_miller@bio-rad.com
Chempetitive Group
Ken Li
312-532-4675
kli@chempetitive.com
