Anion exchange chromatography is a form of ion exchange chromatography (IEX), which is used to separate molecules based on their net surface charge. Anion exchange chromatography, more specifically, uses a positively charged ion exchange resin with an affinity for molecules having net negative surface charges. Anion exchange chromatography is used both for preparative and analytical purposes and can separate a large range of molecules, from amino acids and nucleotides to large proteins. Here, we focus on the preparative anion exchange chromatography of proteins.
Related Topics: Cation Exchange Chromatography, Ion Exchange Chromatography, Types of Chromatography, Column Chromatography Methods and Instrumentation, Medium-Pressure Chromatography
Page Contents
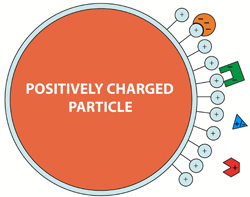
Fig. 1. Schematic diagram of an anion exchange media particle.
A protein’s net surface charge changes with pH in a manner that is dictated by a protein’s isoelectric point, or pI. At a pH equal to a protein’s pI, the protein carries no net charge. At a pH below the pI, the protein carries a net positive charge. If the buffer pH is raised above a protein’s pI, it carries a net negative charge.
Because a protein’s pI is determined by its primary amino acid sequence and can thus be calculated, a buffer can then be chosen that ensures a known net charge for a protein of interest. A positively charged anion exchange resin is thus chosen when the protein of interest carries a net negative charge at the working pH. Proteins with different pI values will have varying degrees of charge at a given pH and thereby have different affinities for the positively charged surface groups on the particles of the anion exchange media; therefore, different proteins will bind to the resin with different strengths, facilitating their separation.
At a particular loading buffer pH, all appropriately charged proteins will bind the resin. For example, if an anion exchange resin is used at a pH of 7.5, in general, all proteins that have a pI <7.5 will carry a net negative charge and will bind the positively charged resin. A salt gradient is then used to separate the protein of interest from other bound proteins — proteins will be eluted in an order depending on their net surface charge. Proteins with pI values closer to 7.5 will elute at a lower ionic strength, and proteins with very low pI values will elute at a high salt concentration.
All ion exchange chromatography relies on electrostatic interactions between the resin functional groups and proteins of interest; thus, the workflow below is given as a generalized IEX workflow, and particular running conditions for anion exchange chromatography may be adjusted to best suit your protein of interest, the buffer system, and the anion exchange resin chosen. Because buffer pH and ionic strength greatly affect protein binding to the column resin, it is very important to ensure that buffer pH is properly titrated and that appropriate counterions are used.
-
- Buffer preparation
Buffer pH and ionic strength are crucial for all forms of ion exchange chromatography. It is best to readjust buffer pH after adjusting salt concentration and ensure that buffer counterions are compatible. Buffer counterions should have the same charge as the resin; for positively charged anion exchange resins, Tris buffers are an excellent choice.
-
- Column equilibration
Equilibrate the column until pH and conductivity readings stabilize (typically requires ≥5 column volumes of buffer).
-
- Sample loading
Because ionic strength and pH are main determinants of protein binding to IEX resins, whenever possible, load the sample in the starting buffer.
-
- Column washing
Wash the column in loading buffer (0% Buffer B) until no protein is detected in the flowthrough (3–5 column volumes).
-
- Elution
Protein can be eluted either by a linear gradient elution or using a step isocratic elution. Often, a gradient elution may be used to optimize elution conditions. Once the elution profile of the protein of interest has been established and it is known at what ionic strength or pH a protein elutes, a step elution can be used to speed the purification process.
|
Gradient Elution
|
Step Elution
|
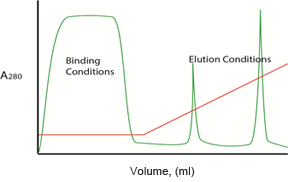
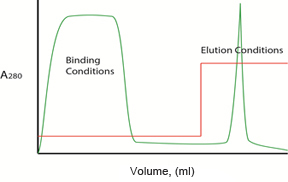
Fig. 2. Gradient vs. step elution. Plots illustrating protein separation with a typical anion exchange column when the A/B buffer ratio is changed in either a linear gradient (left) or stepwise (right) isocratic manner. Protein concentration in the effluent is reported as A280 ( ). Buffer composition during the binding and elution phases is shown as percentage B (
). Buffer composition during the binding and elution phases is shown as percentage B ( ).
).
Note: pH gradients are generally not effective for ion exchange elution because it is very difficult to generate reliable linear pH gradients without altering ionic strength. If pH is used for elution, step gradients may provide more consistent results.
-
- Column Stripping and Equilibration
After the protein of interest has been eluted, proteins that remain bound to the column resin are eluted by increasing the ionic strength or altering the pH of the elution buffer. After all remaining protein has been eluted from the resin, equilibrate the column in low ionic strength buffer. A good choice would be the starting buffer for purification if the column will be used in the near future. A common way to prevent microbial growth during long-term storage is to exchange the column buffer for 20% ethanol in water. Refer to the resin/column manufacturer for storage recommendations.
Anion Exchanger vs. Cation Exchanger
The net charge of the molecule(s) of interest determines resin choice. If positively charged molecules are to be immobilized by the column resin, a cation exchange resin is chosen, whereas a positively charged anion exchange resin is chosen if negatively charged molecules are to be captured by the resin.
For applications such as removing negatively charged DNA or endotoxins, a strong anion exchange column such as Bio-Rad’s ENrich Q chromatography column is an excellent choice. A negatively charged cation exchange resin would not immobilize and cannot remove DNA or endotoxins from the sample.
Unlike DNA, proteins are zwitterionic and thus can carry either a net positive or a net negative charge. Buffer pH will dictate the protein’s net charge. In theory, each protein could be purified using either a cation or an anion exchanger. In practice, proteins are not stable at every pH — a pH that would render a protein of interest positively charged might also denature the protein. Protein stability and buffer choice therefore dictate IEX media choice for protein purification.
Anion Exchange Resin Application Guide
Bio-Rad offers a wide variety of anion exchange resins both in prepacked column form and as bulk media that can be packed into empty chromatography columns. Choose the anion exchange resin that meets your needs from the table below, or contact technical support for assistance.
| Suitability** | |||||
| Media Type | Packaging Format* |
Analytical Scale |
Pilot/ Preparative Scale |
Process Scale |
Application |
| AG 1 | B, GC | ++++ | ++ | + | Strong exchanger. Separation of low MW peptides, nucleotides, inorganic ions using different crosslinkages; high selectivity for anions such as chloride; gravity or low-pressure use |
| AG MP-1M | B, GC | ++++ | +++ | + | Strong exchanger. Macroporous, equivalent to AG 1 for MW >1,000,000; gravity or low pressure use |
| Bio-Rex 5 and AG 4-X4 |
B, GC | ++++ | ++ | Weak exchanger. Used to remove organic acids from sugars; adsorption of mineral acids; gravity or low-pressure use | |
| UNO Q | MPC | ++++ | Strong exchanger. High-resolution biomolecule separation at high flow rates; pH stability 2–12 | ||
| Macro-Prep High Q |
B, C | +++ | ++++ | + | Strong exchanger. High-capacity biomolecule separation; unique surface chemistry allows contaminant removal; pH stability 1–10 |
| Macro-Prep 25 Q | B | ++++ | ++++ | + | Strong exchanger. Similar to Macro-Prep High Q but 25 μm particle size allows higher-resolution separation; unique surface chemistry allows contaminant removal; pH stability 1–10 |
| Macro-Prep DEAE | B, C | +++ | ++++ | + | Weak exchanger. High-capacity biomolecule separation; unique surface chemistry allows contaminant removal; pH stability 1–10 |
| UNOsphere Q | B, C | ++++ | ++++ | ++++ | Strong exchanger. High-productivity, high-capacity biomolecule separation; pH stability 1–14 |
| Aminex | HPLC | ++++ | High-pressure separation of carbohydrates, sugars, and small organic molecules; delivers industry-standard performance (U.S. Pharmacopeia) | ||
| Nuvia Q | B, F | ++++ | ++++ | ++++ | Strong exchanger. Similar to UNOsphere Q but surface modification allows extremely high-capacity biomolecule separation; pH stability 1–14 |
| ENrich Q | MPC | +++ | ++++ | + | Strong exchanger. High-resolution biomolecule separation at high flow rates; pH stability 2–14 |
* B, bottle; C, cartridge (1 ml or 5 ml); GC, gravity column; SC, spin column; HPLC, high-pressure column; MPC, medium-pressure column.
** +, low suitability; ++, moderate suitability; +++, suitable; ++++, high suitability.