StarBright Blue 520 Fluorescent Secondary Antibodies Offer Best Sensitivity among Fluorescent Dye–Labeled Secondary Antibodies That Emit in the Green Channel
StarBright Blue 520 Fluorescent Secondary Antibodies are part of Bio-Rad’s fluorescent western blotting workflow for obtaining reliable, high-quality results.
HERCULES, Calif. — May 09, 2018 — Bio-Rad Laboratories Inc. (NYSE: BIO and BIOb) today announced the launch of StarBright Blue 520 Fluorescent Secondary Antibodies, fluorescent dye–labeled secondary antibodies for use in multiplex western blotting. They exhibit a two- to threefold lower limit of detection than traditional green emission fluorophore-labeled antibodies such as Alexa Fluor 488 or DyLight 488, the current industry standards.
Using traditional chemiluminescent methods to detect and quantify multiple proteins simultaneously can be challenging. Scientists often resort to cutting or reprobing blots, which introduces variability and results in protein loss, thereby rendering the blot irreproducible and unsuitable for quantitation. An alternate method involves attempting to sequentially probe a single blot with multiple fluorescent antibodies to distinguish the signals from each one; however, these protocols are cumbersome to optimize and execute.
To address these workflow challenges, Bio-Rad created the StarBright Blue line of secondary antibodies that enable highly sensitive fluorescent detection, short exposure times, and easy multiplexing for western blotting. These plug-and-play antibodies allow simultaneous detection of up to three proteins (two targets of interest and one housekeeping protein) on the same blot when used with the hFAB Rhodamine Housekeeping Protein Fluorescent Primary Antibodies. The antibodies work seamlessly on nitrocellulose or low-fluorescence PVDF membranes and all other aspects of the standard western blotting workflow remain unchanged.
The StarBright Blue 520 Fluorescent Secondary Antibodies are labeled with a particularly bright fluorescent dye, resulting in short exposure times and a high signal-to-noise ratio. This property stems from the presence of multiple donor-acceptor pairs in each polymer molecule, which enable it to efficiently absorb and emit light. The product’s 520 nm emission wavelength means the StarBright Blue 520 Fluorescent Secondary Antibody can pair with a StarBright Blue 700 Fluorescent Secondary Antibody or with other traditional fluorescent antibodies. It conjugates to highly cross-adsorbed IgG, which leads to low nonspecific binding.
StarBright Blue 520 Fluorescent Secondary Antibodies deliver unmatched sensitivity.
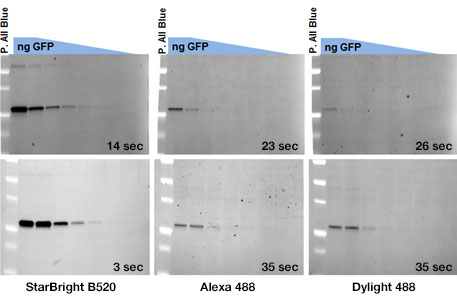
Comparison of StarBright Blue 520 Fluorescent Secondary Antibodies to traditional green emission fluorophores Alexa Fluor 488 and DyLight 488 labeled antibodies (top: goat anti-mouse conjugates, bottom: goat anti-rabbit conjugates).
The StarBright Blue 520 Fluorescent Secondary Antibodies can detect low-abundance protein targets in exposure times that are two to four times shorter than those of traditional fluorescent antibodies.
The release of the StarBright 520 Fluorescent Secondary Antibodies completes Bio-Rad’s high-sensitivity triplex western blotting solution. StarBright Blue 700 and StarBright Blue 520 Fluorescent Secondary Antibodies, and the hFAB Rhodamine Housekeeping Protein Primary Antibodies are part of Bio-Rad’s family of instruments, reagents, and antibodies created to obtain fluorescent western blot results reliably, reproducibly, and with high-quality data. With its recently launched ChemiDoc MP Imaging System, one of the most sensitive and flexible fluorescence detection imaging systems available, and a portfolio of unique fluorescence-labeled secondary and primary antibodies, Bio-Rad is the only life science company to offer a complete western blotting solution.
For researchers new to western blot imaging, Bio-Rad continues to lead the industry in customer support and online resources, including Western Blot Doctor Troubleshooting Guide and a library of videos.
Please visit www.bio-rad.com/starbright520 for more information about Bio-Rad’s StarBright 520 Fluorescent Secondary Antibodies.
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions.
All trademarks used herein are the property of their respective owner.
About Bio-Rad
Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb) is a global leader in developing, manufacturing, and marketing a broad range of innovative products for the life science research and clinical diagnostic markets. With a focus on quality and customer service for over 65 years, our products advance the discovery process and improve healthcare. Our customers are university and research institutions, hospitals, public health and commercial laboratories, biotechnology, pharmaceutical, as well as applied laboratories that include food safety and environmental quality. Founded in 1952, Bio-Rad is based in Hercules, California, and has a global network of operations with more than 8,000 employees worldwide. Bio-Rad had revenues exceeding $2.1 billion in 2017. For more information, please visit www.bio-rad.com.
This release may be deemed to contain certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements we make regarding our development and launch of new products and our expectations regarding our products. Forward-looking statements generally can be identified by the use of forward-looking terminology such as "plan", "believe," "expect," "anticipate," "may," "will," "can," "intend," "estimate," "continue," or similar expressions or the negative of those terms or expressions, although not all forward-looking statements contain these words. Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. These risks and uncertainties include our ability to develop and market new or improved products, product quality and liability issues, our ability to compete effectively, and international legal and regulatory risks. For further information regarding our risks and uncertainties, please refer to the "Risk Factors" and "Management’s Discussion and Analysis of Financial Condition and Results of Operation" in Bio-Rad’s public reports filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K and our Quarterly Report on Form 10-Q. Bio-Rad cautions you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. We disclaim any obligation to update these forward-looking statements.
Press Contact:
Bio-Rad Laboratories, Inc.
Poulomi Acharya
Poulomi_acharya@bio-rad.com
510-741-4507
CG Life
Ken Li
312-532-4675
kli@cglife.com
