- Introduction to Western Blotting
- Transfer Conditions
This section provides an overview of the transfer conditions required for performing electrophoretic protein transfer. It also provides some troubleshooting tips for electrophoretic transfer and outlines a general workflow for the protein transfer from gels to membranes. Find procedures for reagent and materials preparation for protein transfer, tank blotting and semi-dry blotting procedures, and microfiltration in the Protocols section below.
Related Topics: Protein Blotting Equipment, Membranes, Transfer Buffers, and Protein Detection and Imaging.
Page Contents
Overall, the procedures and principles for semi-dry and tank transfers are the same. Gels and membranes are pre-wet and equilibrated with transfer buffer, and the gel/membrane sandwich is placed into the transfer apparatus in the correct orientation to ensure transfer of proteins to the membrane. Additionally, the appropriate power conditions must also be selected.
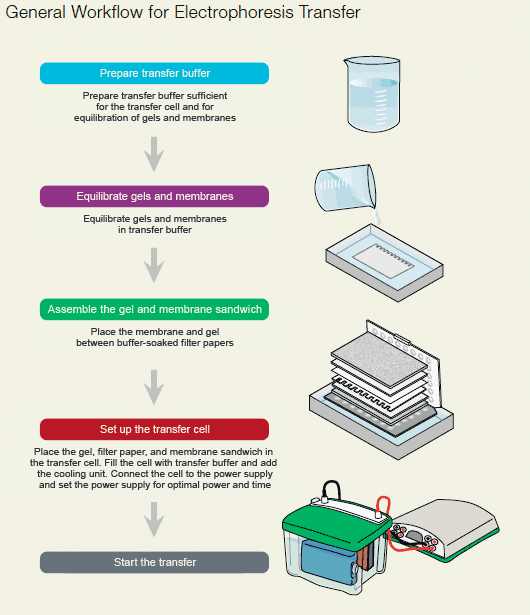
Protein blotting workflow.
Different transfer apparatuses, when used with different gel and buffer systems, require different power settings. The table below provides general guidelines for the voltage and current settings recommended for selected gel and buffer systems. Transfer times are increased for gradient gels and decreased for low molecular weight proteins. The values presented in the table are guidelines — transfer conditions should be optimized for every transfer application. Cooling is generally required for all high-intensity transfers (except when using the Trans-Blot® SD cell) and is recommended for long, unsupervised runs.
Guide to power settings for different gel types.
| SDS-PAGE Gels (Towbin Buffer) | ||
| Low Intensity (time) | High-Intensity (time) | |
| Trans-Blot® cell | ||
| Plate electrodes | 10 V/100 mA, 16 hr | 50–100 V/700–1,600 mA, 30–60 min |
| Wire electrodes | 30 V/100 mA, 16 hr | 100–200 V/300–800 mA, 30 min–4 hr |
| Trans-Blot® Plus cell | 30 V/0.5 A, 16 hr | 100 V/1,500 mA, 60 min |
| Mini Trans-Blot® cell | 30 V/90 mA, 16 hr | 100 V/350 mA, 60 min |
| Criterion™ blotter | ||
| Plate electrodes | 10 V/50–80 mA, 16 hr | 100 V/750–1,000 mA, 30 min |
| Wire electrodes | 10 V/30–40 mA, 16 hr | 100 V/380–500 mA, 60 min |
| Trans-Blot® SD cell | N/A | Mini gels: 10–15 V/5.5 mA/cm2, 10–30 min Large gels: 15–25 V/3 mA/cm2, 30–60 min |
| Trans-Blot® Turbo™ transfer system | N/A | Mini gels: 25 V/1,300 mA, 7 min Midi gels: 25 V/2,500 mA, 7 min |
| Isoelectric Focusing Gels, Native Gels, Basic Proteins, and Acid-Urea Gels (0.7% acetic acid) | ||
| Low Intensity (time) | High-Intensity (time) | |
| Trans-Blot cell | ||
| Plate electrodes | 15 V/200 mA, 16 hr | 30–60 V/600–1,000 mA, 30–60 min |
| Wire electrodes | 30 V/200 mA, 16 hr | 100–150 V/550–850 mA, 30 min–4 hr |
| Trans-Blot Plus cell | 10–30 V/0.15–0.55 A, 16 hr | 100–125 V/1.9–2.4 A, 15–60 min |
| Mini Trans-Blot cell | 30 V/10 mA, 16 hr | 100 V/350 mA, 1 hr |
| Criterion blotter | ||
| Plate electrodes | 10 V/50 mA, 16 hr | 100 V/980–1,200 mA, 30 min |
| Wire electrodes | 10 V/50 mA, 16 hr | 100 V/500–800 mA, 30 min |
| Trans-Blot SD cell | N/A | Mini gels: 10–15 V/5.5 mA/cm2, 10–30 min Large gels: 15–25 V/3 mA/cm2, 30–60 min |
| Trans-Blot Turbo transfer system | N/A | Mini gels: 25 V/1,300 mA, 7 min Midi gels: 25 V/2,500 mA, 7 min |
Electrophoretic Transfer
| Problem | Cause | Solution |
| Poor electrophoretic transfer; bands appear weak on blot (ensure proteins have been transferred by staining both the gel and blot with a total stain. For example, stain the gel with Bio-Safe™ Coomassie or SYPRO Ruby stain, and stain the blot with Ponceau S stain). Alternatively, one could use stain-free technology and LF PVDF membranes | Power conditions were inadequate or transfer time too short |
|
| Power conditions were too high or transfer time too long (proteins may transfer through the membrane and into the filter paper) |
|
|
| Transfer buffer was incorrect or prepared incorrectly |
|
|
| Proteins moved in the wrong direction (the gel/membrane sandwich may have been assembled in the wrong order, the cassette inserted in the tank in the wrong orientation, or polarity of the connections may be incorrect) |
|
|
| The charge-to-mass ratio is incorrect (native transfers) |
|
|
| Protein precipitated in the gel |
|
|
| The power supply circuit is inoperative or an inappropriate power supply was used |
|
|
| The gel percentage was too high (decreasing %T or %C increases gel pore size and increases transfer efficiency) |
|
|
| Regions of poor protein binding on the blot | The membrane was not uniformly wet before transfer |
|
| Buffer tank not filled to correct level |
|
|
| Swirls or missing bands; bands appear diffuse on the blot | Contact between the membrane and the gel was poor; air bubbles or excess buffer remain between the blot and gel |
|
| White spots on membrane | The membrane was not properly wetted or had dried out |
|
| Broad or misshapen bands | Poor gel electrophoresis |
|
| Gel cassette pattern transferred to blot | Foam pads are contaminated or too thin |
|
| Excessive amounts of protein were loaded on the gel or too much SDS was used in the transfer buffer. Proteins can pass through the membrane without binding and recirculate through tank blotting systems |
|
|
| The transfer buffer was contaminated |
|
|
| Overall poor binding to the membrane | Methanol in the transfer buffer is restricting elution |
|
| SDS in the transfer buffer reduces the binding efficiency of proteins |
|
|
| Proteins passed through the membrane. Proteins <15 kD may show decreased binding to 0.45 µm membranes |
|
Videos
This video demonstrates how the Trans-Blot Turbo system measures up against traditional blotting techniques both in terms of equipment and supplies needed and speed.
This instructional video highlights the main features of the Trans-Blot Turbo system as well as the easy assembly and transfer process. Helpful tips are provided along the way to ensure blotting success.
This video demonstrates how the Trans-Blot Turbo system measures up against traditional blotting techniques both in terms of equipment and supplies needed and speed.
This tutorial shows how to use Bio-Rad's Mini-PROTEAN TGX precast gels with the Mini-PROTEAN Tetra cell. Mini-PROTEAN TGX precast gels are long shelf life precast gels that use standard Tris/Glycine buffers.
This tutorial describes the features of Bio-Rad's PowerPac family of power supplies and compares their specifications. It also offers information about parameter selection, common error messages, and available PowerPac accessories. Important safety information is also provided.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6211 | Transfer Buffers Formulation | Click to download |
| 6212 | Reagent and Materials Preparation | Click to download |
| 6213 | Tank Blotting Procedure | Click to download |
| 6214 | Semi-Dry Blotting Procedure | Click to download |
| 6215 | Microfiltration | Click to download |



