Several techniques are available to modify gene expression in stem cells, including transfection of nucleic acids into cells. Transfection methods comprise electroporation, lipid-mediated delivery, and biolistic particle delivery, as well as cell transduction though viral-mediated gene delivery.
Transfection is used to reprogram somatic cells to induced pluripotent stem cells (iPSCs), and to engineer stem cells for therapeutic or research purposes. In addition to facilitating gene expression, transfection and transduction techniques can be used to downregulate gene expression levels via RNA interference (RNAi).
Stem cells can be difficult to transfect. The methods used for stem cell transfection vary with cell type, species, the molecule being delivered, and the intended downstream application. Electroporation and lipid-mediated delivery have been the most common methods for the transfection of stem cells. Biolistic particle delivery is a cell-type independent technique that can be used to transfer genetic materials into stem cells. The challenges of chemical and physical transfection methods have led many researchers to adopt viral-transduction methods for gene delivery in stem cells.
Related Topics: Stem Cell Research, Isolation and Maintenance of Stem Cells, Differentiation of Stem Cells, Analysis of Stem Cells, and Stem Cells in Therapeutics and Research.
Page Contents
Electroporation temporarily disrupts the plasma membrane lipid bilayer with an electric shock, allowing molecules to enter the cell. Electroporation protocols are optimized for various cell types. Bio-Rad has determined the optimal electroporation protocols for delivery of materials for most common cell types and cell lines. The Transfection Protocol Library contains standard and customized user-submitted protocols for a wide variety of cells (select cell type and protocol type from the dropdown menus).
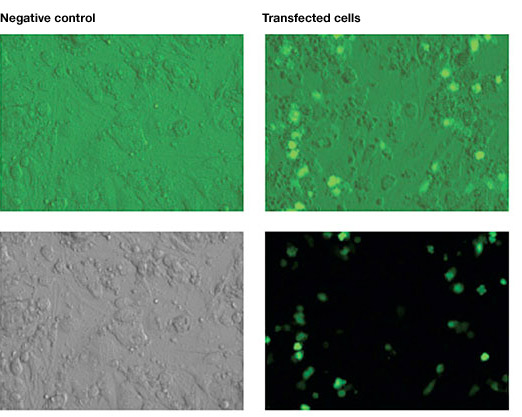
Mouse ESC, cultured on STO mouse embryonic fibroblast feeder (MEF), were electroporated using the Gene Pulser Xcell™ Electroporation System with an exponential waveform at 240 V and 75 µF.
Lipid-mediated transfection uses cationic lipids to deliver DNA and RNA into cells. Transfectin™ Lipid Reagent, a mixture of cationic lipids and a co-lipid DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) has been used to transfect stem cells such as neural stem cells (Sun et al. 2010, Lang et al. 2012).
In lipid-mediated transfection, lipid is mixed with nucleic acids and then added directly to the cells. Lipid-encapsulated molecules are thought to enter the cell via endocytosis. Transfection efficiency varies depending on the reagent and the cell type. Lipid toxicity can sometimes be a problem when transfecting cells. Transfectin Lipid Reagent has low toxicity and is effective in stem cell transfection protocols.
Biolistic particle delivery is the injection of small compounds, proteins, or nucleic acids into cells by coating them onto inert micron-sized particles, which are shot directly into cells as microprojectiles using a high-pressure burst of helium.
The Helios® Gene Gun and PDS-1000 / He™ and Hepta™ Systems use a helium pulse to accelerate gold or tungsten particles coated with DNA, RNA, or protein into target cells. Biolistic delivery entails three steps: coating microparticles with DNA (or other transfectant), drying them onto a macrocarrier disk, and propelling them into the target cells. The macrocarrier disk is accelerated with high-pressure helium into a stopping screen, which frees the microprojectiles to bombard the cells.
Like the other techniques, optimization is required for cell type and source. Optimization includes the density of cells plated, the quantity of microparticles used, the pressure used to deliver the particles, and the amount of vacuum applied. A major advantage of the biolistic approach is the ability of the particles to be carried through many layers of cells, for example, effectively transfecting 3D cultures or embryoid bodies.
Other advantages of biolistic particle delivery systems include the ability to codeliver more than one plasmid or other nucleic acid, their effectiveness with all cell types, the fact that no carrier is needed, the low levels of transforming DNA required, and their additional use for the delivery of proteins and RNA, including modified RNAs such as miRNA mimics.
Viral gene delivery, or transduction, was the first method used to introduce genes into somatic cells to reprogram them to become iPSCs (Takahashi and Yamanaka 2006, Okita et al. 2007, Takahashi et al. 2007, Wernig et al. 2007).
Gamma-retroviruses have commonly been used for cellular reprogramming; however, the lack of control over the gene integration site has led to the development of protocols using different viral vectors. A polycistronic doxycycline-inducible lentivirus that reduces the frequency of integration events while still producing multiple proteins has been engineered (Carey et al. 2009), lowering the risk of deleterious proviral insertion. Although viral-mediated gene delivery can be effective, the production of the virus itself is time consuming. Lentivirus production is accomplished using three separate constructs — a transfer vector, a packaging plasmid, and an "envelope" plasmid. The addition of enzyme inhibitors, micro RNA (miRNA), and miRNA mimics have been found to increase lentiviral transduction efficiency (Anokye-Danso et al. 2012). Sendai virus, an RNA virus (Fusaki et al. 2009, Seki et al. 2012), and adenovirus (Stadfield et al. 2008), neither of which integrate into host genomes, have also been used to reprogram somatic cells.
Anokye-Danso F et al. (2012). How microRNAs facilitate reprogramming to pluripotency. J Cell Sci 125, 4179–4187. PMID: 23077173
Carey BW et al. (2009). Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci USA 106, 157–162. PMID: 19109433
Fusaki N et al. (2009). Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser, B Phys Biol Sci 85, 348–362. PMID: 19838014
Lang F-Y et al. (2012). Genome-wide profiling identified a set of miRNAs that are differentially expressed in glioblastoma stem cells and normal neural stem cells. PLoS One 7, e36248. PMID: 22558405
Okita K et al. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317. PMID: 17554338
Seki T et al. (2012). Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat Protoc 7, 718–728. PMID: 22422317
Stadfield M et al. (2008). Induced pluripotent stem cells generated without viral integration. Science 322, 945–949. PMID: 18818365
Sun G et al. (2010). Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol 30, 1997–2005. PMID: 20123967
Takahashi K and Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. PMID: 16904174
Takahashi K et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. PMID: 18035408
Wernig M et al. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324. PMID: 17554336
Zsigmond E (2009). Transfection of mouse and human embryonic stem cells by electroporation using the Gene Pulser MXcell™ system. Bio-Rad Bulletin 5904.
