
Transfection generally refers to the introduction of foreign DNA into bacterial and/or mammalian cells. Transfection is an important tool used in studies investigating gene function and the modulation of gene expression, thus contributing to the advancement of basic cellular research, drug discovery, and target validation. This section provides an overview of different transfection methods, transfection workflow, factors affecting transfection efficient, and protocols.
Related Topics: Posttransfection Analysis of Cells, Instrument-Based Transfection Methods, and Chemical and Viral Transfection.
Page Contents
Transfection can be accomplished using chemical, biological, or physical methods. Common methods include electroporation, the use of a virus vector, lipofection, and biolistics. Many types of genetic material, including plasmid DNA, siRNA, proteins, dyes, and antibodies, may be transfected using any of these methods. However, a single method cannot be applied to all types of cells; transfection efficiencies and cytotoxicity may vary dramatically and depend on the method, cell type being utilized, and types of experiments being performed. Therefore, to obtain high efficiencies, all relevant factors should be considered for planning and selecting the appropriate transfection method.
| Method | Function | Recommended Cells | Products |
| Electroporation | Nucleic acids or other molecules are introduced into cells by creating transient pores in the plasma membrane using an electric pulse | Eukaryotic cells (primary, stem cells), prokaryotic cells (bacteria, yeast), plant protoplasts | Gene Pulser Xcell™ electroporation system Gene Pulser MXcell™ electroporation system MicroPulser™ electroporator |
| Lipid-mediated | Uses lipids to cause a cell to absorb nucleic acids; transfer of genetic material into the cell takes place via liposomes, which are vesicles that can merge with the cell membrane | Immortal cells, adherent (attached), or suspension cells | TransFectin lipid reagent SiLentFect lipid reagent |
| Biolistic particle delivery | Delivery of nucleic acids into cells via high velocity nucleic acid-coated microparticles | Plant, primary cells, tissue, and in vivo applications | Helios™ gene gun PDS-1000/He™ biolistic particle delivery system |
| Viral vector (for example, retrovirus, lentivirus, adenovirus, or adeno-associated viruses) | Uses viral vectors to deliver nucleic acids into cells | Attached adherent cells, stem cells, primary cells |
Successful transfection is usually measured in terms of transfection efficiency and cell viability — the higher the efficiency and viability, the better the transfection. Several important factors, such as the DNA quantity and quality, cell type, cell health, and transfection method (as stated above) affect transfection results. Transfection efficiency, for example, varies greatly with the cell type and its physiological condition prior to transfection. Ideally, the cells should be actively growing, healthy, and free of contamination.
Another way to present factors impacting transfection results is to consider the entire transfection workflow and the series of key sub-experiments it comprises. Each sub-experiment can significantly affect efficiency and viability. The typical workflow for a transfection experiment is as follows:
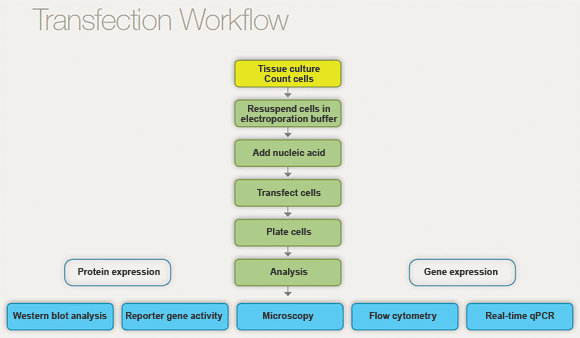
Typical workflow for a transfection experiment.
The following table summarizes the factors to consider for efficient transfection:
| Cell Health |
Cells should be grown in medium appropriate for the cell line, supplemented with serum or growth factors as needed for viability
|
| Confluency |
Transfect cells at 40–80% confluency (cell type dependent)
|
| Passages of DNA |
Number of Passages (cell type dependent)
DNA Quality and Quantity
|
| Time/Serum |
Time
Serum
|
The transfection protocol online library contains protocols obtained from the literature, developed by Bio-Rad scientists, or submitted by scientists like you. Browse protocols to view our library and find your starting point or submit a protocol by clicking the proper technology.
Belyansteva IA (2009). Helios Gene Gun-mediated transfection of the inner ear sensory epithelium. Methods Mol Biol 493, 103–123. PMID: 18839344
Fujiki R et al. (2009). GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature 459, 455–459. PMID: 19377461
Helledie T et al. (2008). A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem Cells Dev 17, 837–848. PMID: 18752428
Hockemeyer D et al. (2009). Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 27, 851–857. PMID: 19680244
Huang B et al. (2008). RNA interference-mediated in vivo silencing of fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum Gene Ther 19, 763–773. PMID: 18627219
Shimamura K et al. (2007). Generation of secondary small interfering RNA in cell-autonomous and non-cell autonomous RNA silencing in tobacco. Plant Mol Biol 63, 803–813. PMID: 17225952
Su L et al. (2009). Neural stem cell differentiation is mediated by integrin beta4 in vitro. Int J Biochem Cell Biol 41, 916–924. PMID: 18834954
Tseng CN et al. (2013). A method to identify RNA A-to-I editing targets using I-specific cleavage and exon array analysis. Mol Cell Probes 7, 38–45. PMID: 22960667
Related Content
Videos
This video protocol describes the preparation of primary hematopoietic cell cultures from murine bone marrow for electroporation.
This tutorial highlights the main components and features of the Gene Pulser Xcell system. It provides information about system installation and the setup of electroporation experiments, including important troubleshooting tips and answers to frequently asked questions. Ordering information for system components and accessories is also provided.
Documents
TEST
| 6176 | Electroporation Systems Overview | Click to download |
| 6177 | Biolistic Particle Delivery Systems | Click to download |
| 6178 | Recommended Biolistic System by Cell Types | Click to download |
| 6179 | Lipid Transfection Reagents Selection Guide | Click to download |





