- Life Science
- Drug Discovery and Development
- Biochemical and Cellular Assays
- Biosimilar Development
- Clinical Research
- Instrument Validation and CFR-Compliant Software
- Lead Discovery: Screening and Optimization
- Pharmaceutical Manufacturing and Release Testing
- Preclinical Research
- Process Development and Scale-Up
- Technical Support for Drug Discovery and Development Researchers
- Target Discovery: Identification and Validation
- Viral Vector Vaccine Development
Bio-Rad offers products and services to accelerate the drug discovery and development journey, from target identification through scale-up to manufacturing. We understand that consistency, reproducibility, and reliability are critical to your research, so our products are developed and manufactured to the highest quality standards. We know that maintaining high productivity is a constant challenge, so our products are easy to use and designed to automate steps of your workflow, reducing hands-on time and freeing you to focus on your most critical tasks.
 Bio-Rad is ISO-certified with cGMP facilities set up to ensure our products meet the stringent requirements of regulatory agencies. We have experience working directly with the FDA and regularly assist our customers with audit requirements. We offer solutions that are customizable to fit perfectly with your needs in drug discovery, development, and manufacturing and help you drive efficiency in target identification, candidate screening, assay development, and protein or antibody production, from small scale to large scale.
Bio-Rad is ISO-certified with cGMP facilities set up to ensure our products meet the stringent requirements of regulatory agencies. We have experience working directly with the FDA and regularly assist our customers with audit requirements. We offer solutions that are customizable to fit perfectly with your needs in drug discovery, development, and manufacturing and help you drive efficiency in target identification, candidate screening, assay development, and protein or antibody production, from small scale to large scale.
Page Contents
Stages of Drug Discovery and Development
|
Target Discovery: Identification and Validation |
Preclinical Research |
Process Development and Scale-Up |
|
Lead Discovery: Screening and Optimization |
Clinical Research |
Pharmaceutical Manufacturing and Release Testing |
Bio-Rad's Drug Discovery and Development Workflow Solutions
Learn how Bio-Rad's products align with your drug discovery and development workflows.
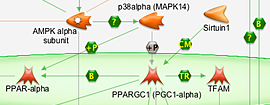
Biochemical and Cellular Assays
In vitro and in vivo discovery approaches with high sample throughput and high-content analysis to characterize biological responses.
Biomarker Discovery & Validation
Biomarker monitoring across drug discovery, development, and clinical stages is key to defining drug effects.
Biologic Development
Biologic development brings patients closer to personalized medicine to treat health problems.
Biosimilar Development
Biosimilar development requires physicochemical and functional characterization to ensure similarity.
Cancer Immunotherapy Development
Learn about trends in cancer immunotherapy development, such as CAR-T, and tools for genomic, cellular, and proteomic research.
CRISPR Gene Editing
CRISPR enables researchers to edit their desired target genes efficiently and accelerate drug development.
Viral Vector Vaccine Development
The COVID-19 pandemic opened the door to expedited vaccine development using new technologies and therapies that have traditionally been used in other applications.
|
|
Technical Support |
|
Instrument Validation and CFR-Compliant Software |
|
|
Expert Care Service Plans |
|
ISO Certificates |










