This section provides an overview of different detection methods used to visualize proteins after immunodetection. The theory behind several commonly used western blotting detection methods such as colorimetric, chemiluminescent and fluorescent methods, and other less common methods, such as chemifluorescence, autoradiography, and immunogold labeling methods are highlighted below.
Related Topics: Antibody Selection and Dilution, Stripping and Reprobing Membranes, and Western Blotting.
Page Contents
Membrane-bound proteins are generally detected using secondary antibodies that are labeled with radioisotopes or colloidal gold, or that are conjugated to fluorescent molecules (fluorophores) or an enzyme such as alkaline phosphatase (AP) or horseradish peroxidase (HRP). Early blotting systems used 125I-labeled reagents similar to those used in radioimmunoassays. These systems provide sensitive results, but the special handling and disposal problems of 125I reagents have discouraged continued use of this technique. Since then, a number of enzyme systems and detection reagents have evolved.
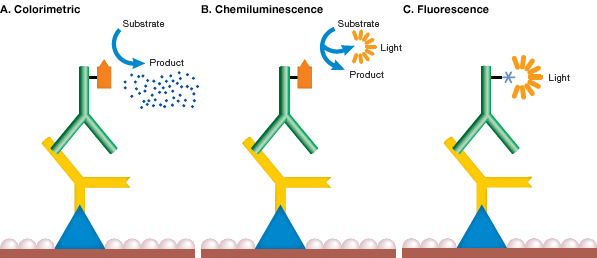
Mechanism of detection chemistries. In each method of western blot detection, a detectable signal is generated following binding of an antibody specific for the protein of interest. In colorimetric detection (A), the signal is a colored precipitate. In chemiluminescence (B), the reaction itself emits light. In fluorescence detection (C), the antibody is labeled with a fluorophore.
The most common detection methods use secondary antibodies conjugated to AP or HRP. In these methods, when the enzyme substrate is added, either a colored precipitate is deposited on the blot (colorimetric detection) or a chemiluminescent or fluorescent product is formed and the light signal is captured on film or with a digital imaging system (see figure above). Secondary antibodies conjugated to fluorophores are gaining popularity and can be directly visualized and captured with a compatible imager, without the need for additional liquid substrate (see Fluorescence Detection).
Colorimetric HRP systems
HRP systems have an advantage over other detection systems in that both the enzyme conjugate and colorimetric detection substrates are economical. The most common substrates for colorimetric HRP are 4-Chloro-1-naphthol (4CN) (Hawkes et al. 1982) and 3, 3'-diaminobenzidine (DAB) (Tsang et al. 1985) (see figure below). Some limitations of HRP colorimetric detection systems are decreased sensitivity when compared to AP colorimetric detection systems, fading of blots upon exposure to light, inhibition of HRP activity by azide, and nonspecific color precipitation.
Enzymes such as AP and HRP convert several substrates to a colored precipitate (see table below). As the precipitate accumulates on the blot, a colored signal develops and is readily visible by eye on the blot. The enzymatic reaction can be monitored and stopped when the desired signal over background is reached. Colorimetric detection is easier to use than film-based detection methods, which require trial and error in determining appropriate exposure times and uses costly materials such as X-ray film and darkroom chemicals. Colorimetric detection is considered a medium-sensitivity method when compared to radioactive or chemiluminescence detection.
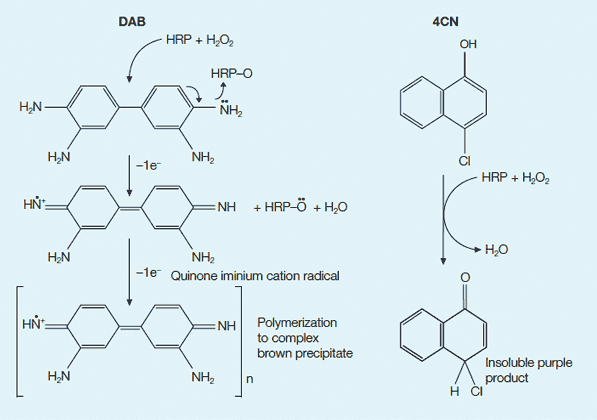
Colorimetric detection options with HRP. DAB and 4CN are commonly used chromogenic substrates for HRP. In the presence of H2O2, HRP catalyzes the oxidation of the substrate into a product that is visible on a blot. Left, reaction with DAB; right, reaction with 4CN.
Colorimetric AP Systems
Colorimetric AP systems use soluble 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) as substrates to produce a stable reaction product that will not fade (see Figure below). AP can easily be inactivated by exposure to acidic solutions. Multiple probing of the same membrane with alternative antibody probes can be performed using substrates that produce different colors, such as blue and red (Blake et al. 1984, Turner 1983, Kurien and Scofield 2003).
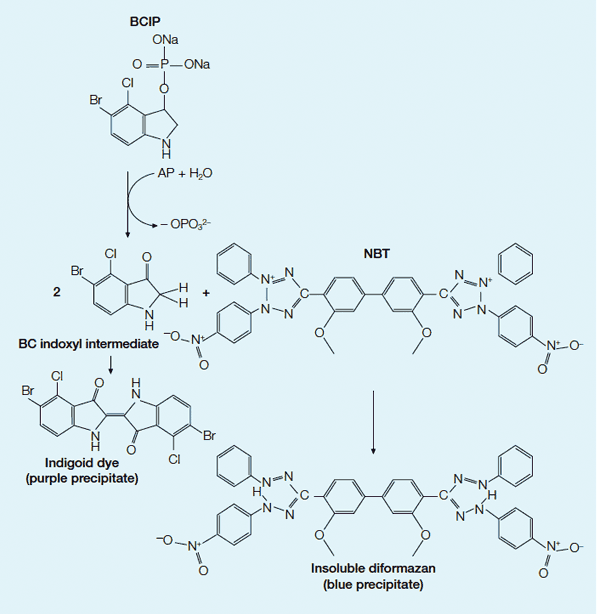
AP colorimetric development. In the colorimetric system, AP catalyzes the substrates BCIP and NBT to produce a colored precipitate visualizing the protein on a western blot. First the dephosphorylation of BCIP by AP occurs, yielding a bromochloro indoxyl intermediate. The indoxyl is then oxidized by NBT to produce an indigoid dye (purple precipitate). The NBT is also reduced by the indoxyl, opening the tetrazole ring to produce an insoluble diformazan (blue precipitate). The combination of the indigoid dye of the BCIP and the insoluble formazan of the NBT forms a purple-blue colored precipitate.
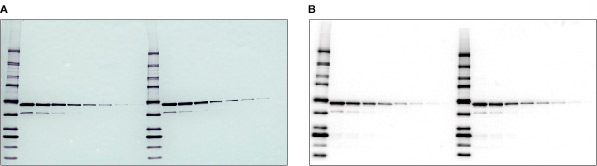
Colorimetric and chemiluminescent blots. A dilution of a GST fusion protein was immunodetected using a monoclonal antibody specific to GST followed by A, an AP-conjugated secondary antibody and BCIP/NBT substrate for colorimetric detection, or B, an HRP-conjugated secondary antibody and Immun-Star™ WesternC™ chemiluminescent substrate for chemiluminescence detection.
Colorimetric detection systems
| Detection Method | Substrate | Detection Sensitivity | Signal Color | Product Options | Advantages | ||
| Detection and Substrate | |||||||
| Immun-Blot Assay Kits | Kits | Dry Powder | |||||
| Colorimetric HRP | 4CN | 500 pg | Purple | X | X | X | Fast color development, low cost, low background enzyme activity |
| Colorimetric HRP | DAB | 500 pg | Brown | X | Insoluble product, readily chelated with osmium tetroxide. Sensitivity can be enhanced further by addition of metals | ||
| Colorimetric HRP | Opti-4CN™ | 100 pg | Purple | X | High sensitivity, nonfading color, low background | ||
| Colorimetric HRP | Amplified Opti-4CN | 5 pg | Purple | X | Best sensitivity available — equal to chemilumine-scence; kit provides all needed components | ||
| Colorimetric AP | BCIP/NBT | 100 pg | Purple | X | X | X | High sensitivity |
Premixed and Individual Colorimetric Substrate
Premixed enzyme substrate kits and development reagents, including powdered 4CN and DAB color development reagents, are also available. The premixed kits are convenient and reliable, and they reduce exposure to hazardous reagents used in the color development of protein blots.
Immun-Blot® Assay Kits
Immun-Blot assay kits provide the reagents required for standard HRP/4CN or AP colorimetric detection on western blots with the added convenience of premixed buffers and enzyme substrates. In addition, these kits contain a secondary antibody conjugated to either HRP or AP. All kit components are individually tested for quality in blotting applications. Included in each kit is an instruction manual with a thoroughly tested protocol and troubleshooting guide that simplifies immunological detection.
Opti-4CN™ and Amplified Opti-4CN Substrate and Detection Kits
Colorimetric HRP detection with 4CN already offers very low background and a detection sensitivity of about
500 pg of antigen. Bio-Rad's Opti-4CN kit improves this detection sensitivity to 100 pg. Opti-4CN is available as a premixed substrate kit or combined with an HRP-conjugated antibody in a detection kit.
Amplified Opti-4CN substrate and detection kits are based on proprietary HRP-activated amplification reagents from Bio-Rad. These kits allow colorimetric detection to 5 pg, matching or even exceeding the sensitivity achieved with radiometric and some chemiluminescence systems, but without the cost or time involved in darkroom development of blots.
Chemiluminescence occurs when a chemical substrate is catalyzed by an enzyme, such as AP or HRP, and produces light as a by-product [see figures above]. The light signal can be captured on X-ray film or by a charge-coupled device (CCD) imager such as the ChemiDoc XRS+ System and ChemiDoc MP System. This technology is easily adapted to existing western blotting procedures because chemiluminescence uses enzyme-conjugated antibodies to activate the light signal. The blocking and wash methods are familiar procedures.
The advantages of chemiluminescent western blotting over other methods are speed and sensitivity (see table below). This method is perfect for CCD imaging, which has typical blot exposure times of 5 sec to 5 min, depending on the sensitivity of the substrate. This is a large improvement over 125I systems, which can require up to 48 hr for film exposure. Detection of protein down to femtogram amounts is possible with these systems. This is more sensitive than most colorimetric systems and comparable to radioisotopic detection. The detection sensitivity depends on the affinity of the protein, primary antibody, secondary antibody, and HRP substrate, and can vary from one sample to another.
Chemiluminescence detection systems
| Detection Method | Substrate | Detection Sensitivity | Product Options | Advantages | Disadvantages |
| Chemiluminescent HRP | |||||
| Immun-Star HRP | Luminol | 1–3 pg | Conjugates | Short (30 sec) exposure | Azide inhibits enzyme activity |
| HRP substrate | Signal duration of 6–8 hr | ||||
| Immun-Blot kits | Compatible with PVDF and nitrocellulose | ||||
| Working solution stable for 24 hr at room temperature | |||||
| Immun-Star WesternC | Luminol | Femtogram | Conjugates | Signal duration up to 24 hr |
Azide inhibits enzyme activity |
| Optimized for CCD imagers | |||||
| High sensitivity | |||||
| Chemiluminescent AP | |||||
| Immun-Star AP | CDP-Star | 10 pg | Conjugates | 30 sec to 5 min exposure | Endogenous phosphatase activity may lead to false positives |
| AP substrate | Signal duration up to 24 hr |
||||
| Immun-blot kits | Blot can be reactivated | ||||
Safety is another advantage of chemiluminescence detection. It does not have the safety concerns associated with isotope detection, such as exposure of personnel to radiation, high costs, and environmental concerns.
Immun-Star™ Chemiluminescence Kits
Immun-Star kits include either CDP-Star substrate (activated by AP) or luminol (activated by HRP) and produce a strong signal on both nitrocellulose and PVDF. The light signal generated with Immun-Star kits not only gives a fast exposure but also lasts for as long as 24 hr (Immun-Star™ WesternC™ Chemiluminescence Kit and Immun-Star™ AP Kits) after initial activation of the blot. These blots can be reactived with fresh substrate, even weeks after the signal has been depleted. They can also be stripped and reprobed multiple times.
The Immun-Star WesternC chemiluminescence kit is designed for use with Precision Plus Protein™ WesternC™ standards and CCD imaging systems. It offers femtogram-level sensitivity and compatibility with any HRP-conjugated antibody (see Figure below). Strong signal intensity optimized for CCD imaging is produced.
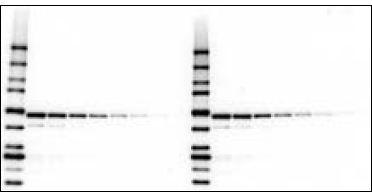
Detection of antigen and Precision Plus Protein WesternC standards using the Immun-Star WesternC chemiluminescence detection kit. Proteins and 5 µl protein standards (lane 1) and a dilution series of an E. coli cell lysate (lanes 2–6) were electrophoresed on a 4–20% Criterion™ gel and transferred to a nitrocellulose membrane. The blot was probed with an antibody specific for GST fusion proteins followed by an HRP-conjugated secondary antibody and StrepTactin-HRP conjugate. After a 5 min incubation in the Immun-Star WesternC detection solution, the blot was imaged on a ChemiDoc XRS+ imager for 5 sec.
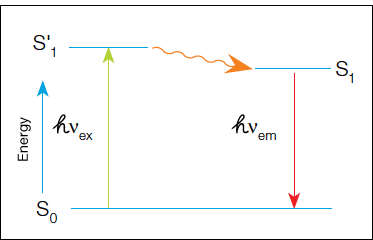
In fluorescence detection, a primary or secondary antibody labeled with a fluorophore is used during immunodetection. A light source excites the fluorophore and the emitted fluorescent signal is captured by a camera to produce the final image. In fluorescence, a high-energy photon (hvex) excites a fluorophore, causing it to leave the ground state (S0) and enter a higher energy state (S'1). Some of this energy dissipates, allowing the fluorophore to enter a relaxed excited state (S1). A photon of light is emitted (hvex), returning the fluorophore to the ground state. The emitted photon is of a lower energy (longer wavelength) due to the dissipation of energy while in the excited state.
When using fluorescence detection, consider the following optical characteristics of the fluorophores to optimize the signal:
- Quantum yield — efficiency of photon emission after absorption of a photon. Processes that return the fluorophore to the ground state but do not result in the emission of a fluorescent photon lower the quantum yield. Fluorophores with higher quantum yields are generally brighter
- Extinction coefficient — measure of how well a fluorophore absorbs light at a specific wavelength. Since absorbance depends on path length and concentration (Beer's Law), the extinction coefficient is usually expressed in cm-1M-1. As with quantum yield, fluorophores with higher extinction coefficients are usually brighter
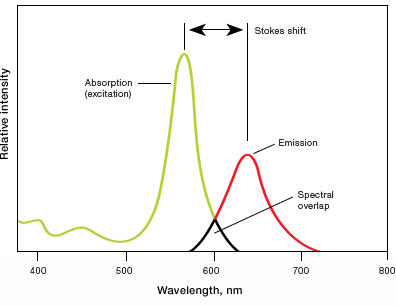
- Stokes shift — difference in the maximum excitation and emission wavelengths of a fluorophore. Since some energy is dissipated while the fluorophore is in the excited state, emitted photons are of lower energy (longer wavelength) than the light used for excitation. Larger Stokes shifts minimize overlap between the excitation and emission wavelengths, increasing the detected signal
- Excitation and emission spectra — excitation spectra are plots of the fluorescence intensity of a fluorophore over the range of excitation wavelengths; emission spectra show the emission wavelengths of the fluorescing molecule. Choose fluorophores that can be excited by the light source in the imager and that have emission spectra that can be captured by the instrument. When performing multiplex Westerns, choose fluorophores with minimally overlapping spectra to avoid channel crosstalk
Several fluorophores spanning a wide range of excitation and emission wavelengths are now available, including some based on organic dyes (for example, cyanine and fluorescein), nanocrystals of semiconductor material, and naturally fluorescent proteins (for example, phycobiliproteins such as phycoerythrin and allophycocyanin).
Fluorescence detection (see figure below) offers several advantages over other methods:
- Multiplexing — use of multiple and differently colored fluorophores for simultaneous detection of several target proteins on the same blot. When detecting multiple proteins in a fluorescent multiplex western blot, ensure the fluorescent signals generated for each protein can be differentiated. Use primary antibodies from different host species (for example, mouse and rabbit) and secondary antibodies that are cross-absorbed against other species to avoid cross-reactivity. Use fluorophores conjugated to secondary antibodies with distinct spectra so they can be optically distinguished from each other to avoid cross-channel fluorescence
- Dynamic range — a 10-fold greater dynamic range over chemiluminescence detection and, therefore, better linearity within detection limits
- Stability — many fluorescent molecules are stable for a long period of time, allowing blots to be stored for re-imaging at a later date – often weeks or months later – without significant signal loss. Most fluorescence techniques are also compatible with stripping and reprobing protocols, provided the blots are not allowed to dry out between successive western blotting detection rounds
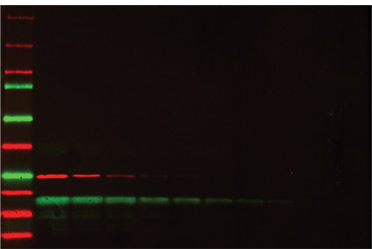
Multiplex fluorescence detection of a two-fold dilution series of two proteins, GST (red) and soybean trypsin inhibitor (green). Starting concentration was 500 ng of each protein. Precision Plus Protein™ WesternC™ standards were used as markers.
Bioluminescence
Bioluminescence is the natural light emission by many organisms. Bioluminescence systems differ in the structure and function of enzymes and cofactors involved in the process as well as the mechanism of the light-generating reactions. Bioluminescence is also used as a detection method for proteins and nucleic acids on a membrane.
Bioluminescence detection involves incubation of the membrane (with bound antigen-antibody-enzyme complex) in a bioluminogenic substrate and simultaneous measurement of emitted light (see figure below). The substrate involved in this detection system is a luciferin based derivative. Light detection is performed using a photon-counting camera and the blotted proteins are visualized as bright spots. This technique is similar to chemiluminescence in its sensitivity and speed of detection, but it is not widely used and few bioluminogenic substrates are commercially available. PVDF is the preferred membrane for bioluminescence detection because nitrocellulose membranes may contain substances that inhibit luciferase activity.
Chemifluorescence
Chemifluorescence is the enzymatic conversion of a substrate to a fluorescent product (see figure below). Fluorogenic compounds (nonfluorescent or weakly fluorescent substances that can be converted to fluorescent products) are available for use with a wide variety of enzymes, including AP and HRP. The enzyme cleaves a phosphate group from a fluorogenic substrate to yield a highly fluorescent product. The fluorescence can be detected using a fluorescence imager such as the PharosFX System or ChemiDoc MP System. Chemifluorescence provides a stable fluorescent reaction product so blots can be scanned at a later time. This method is also compatible with standard stripping and reprobing procedures.
Autoradiography
The gamma-emitting radioisotope 125I can be used to label lysines in immunoglobulins for radiometric antigen detection (see figure below). Direct immunological detection (using labeled secondary antibodies) of as little as 1 pg of dotted immunoglobulin is possible with high specific activity 125I probes. Radiolabeled blots can be detected using X-ray film, a method known as autoradiography. Due to the hazards associated with radiolabeled conjugates, autoradiography is declining in popularity in favor of colorimetric and chemiluminescence methods.
Immunogold Labeling
Immunogold detection methods utilize gold-labeled secondary antibodies for antigen detection. Because this method has relatively low sensitivity and the signal is not permanent, silver enhancement methods similar to those described for colloidal gold total protein stains were developed as a means of enhancing the signal (see figure below). With silver, background is produced on the blot and sensitivity is increased 10-fold, equivalent to colorimetric AP detection and several times more sensitive than autoradiography.
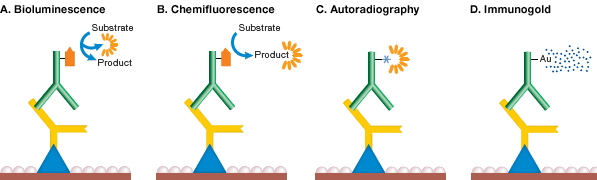
Mechanism of detection chemistries. In bioluminescence detection (A), the enzyme reaction itself emits light, while in chemifluorescence (B), the product of the reaction is fluorescent. In autoradiography (C), the secondary antibody itself carries a radioactive label, and in immunogold labeling (D), the secondary antibody is labeled with gold and signal is enhanced by silver precipitation.
Bayer EA and Wilchek M (1980). The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal 26, 1–45.
Blake MS et al. (1984). A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem 136, 175–179.
Chaiet L and Wolf FJ (1964). The properties of streptavidin, a biotin-binding protein produced by Streptomyces. Arch Biochem Biophys 106, 1–5.
Guesdon J-L et al. (1979). The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem 27, 1131–1139.
Hawkes R et al. (1982). A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119, 142–147.
Hsu SM et al. (1981). Use of avidin-biotin-peroxidase complex (ABC) in immuno-peroxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29, 577–580.
Kurien BT and Scofield RH (2003). Protein blotting: a review. J Immunol Methods 274, 1–15.
Tsang VC et al. (1985). Enzyme-linked immunoelectrotransfer blot (EITB). In Enzyme-Mediated Immunoassay. T.T. Ngo and H.M. Lenhoff, eds. (New York: Plenum Press), pp 389–414.
Documents
TEST
| Number | Description | Options |
|---|---|---|
| 6216 | Detection Buffer Formulations | Click to download |
| 6218 | Blot Stripping & Reprobing | Click to download |
| 6219 | Immunodetection | Click to download |
