
On This Page |
Novel Therapeutics Called Biologics | Focus on Monoclonal Antibodies | Drug Discovery and Development | References | Resources |
Medicines called biologics are transforming the healthcare landscape by offering patients with hard-to-treat conditions a real benefit. Biologics are part of a wave of more targeted therapies that are being developed as drug makers move towards a personalized medicine approach in treating disease.
Novel Therapeutics Called Biologics
Biologics are not a new class of drugs, as biological drugs such as insulin have been available for decades. However, in recent years the number of biological drugs available to patients and the diseases that they treat have grown tremendously. Our increased understanding of the underlying biological processes that inform human health and the changes that happen during disease or chronic illness have helped spur the development of novel biological drugs targeted to provide treatment and relief for patients.1
Biologics are large complex molecules consisting of a combination of proteins, peptides, nucleic acids, sugars, or other cellular structures, produced within living cells The shifting focus toward biological drug products results in targeted treatments that have mechanisms of action previously unavailable from existing small molecule drugs.
Both small and large molecule drugs interact with a patient's biology; however, small molecule drugs often work as inhibitors disrupting a process ordinarily associated with a particular disease. Small molecules have the ability to penetrate cells, with the potential for off-target interactions that can cause side effects. Large biologic drugs, in contrast, are designed to bind with extreme precision to specific targets and in doing so can target molecular processes that small molecule drugs cannot.
Focus on Monoclonal Antibodies
Monoclonal antibody (mAb) biological drugs are the most commonly developed when compared to other biological drug types, such as proteins, enzymes, and vaccines. In terms of approvals for novel medicines, biological drugs have grown to comprise approximately one-third of all new medicines approved by the US FDA.2 In addition, more than half the drugs currently in development by pharma and biotech companies are biologics.
Novel biological drugs targeted towards cancer treatment receive the most R&D funding with rare diseases, autoimmune disorders and neurological disorders, occupying the next three spots.3 In addition to an increase in spending on R&D for biological drugs, supportive government initiatives and demand for personalized medicines are contributing to the expectation that the global market size for mAbs will continue to rise over the next several years.
Manufacturing Monoclonal Antibodies
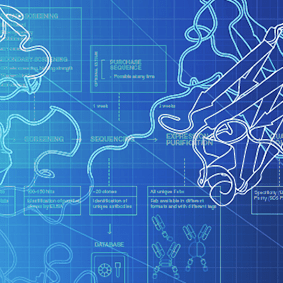
The production of a biological drug is dependent upon a manufacturing process which is time consuming, challenging, expensive, and often complicated. The development of the manufacturing process for a biologic includes genetically engineering a cell to produce the biologic, multiple steps for purification of the biologic through chromatography resins, and associated testing throughout to ensure product quality, purity, and stability.4 The active component of the biologic drug, often a portion or modification of the original protein or polypeptide may not be clearly characterized. Because of the heterogeneous nature of the molecule, the biologic can have an impurity profile that varies with the manufacturing and testing process utilized.
Explore a wide variety of research solutions to accelerate your biologic development. Included are workflow options, applications, technical information, protocols, instruments, and more.
Drug Discovery and Development
Bio-Rad offers products and services to accelerate the drug discovery and development journey, from target identification through scale-up to manufacturing.
References
1 Morrow T (2004). Defining the Difference: What Makes Biologics Unique, Biotechnology Healthcare, pp. 24-29.
2 Gottleib S. Capturing the Benefits of Competition for Patients
3 Hooven M (2017). Opportunities and Challenges in Biologic Drug Delivery, Am Pharmaceutical Review, posted 22 Dec 2017.
4 Jallal, B. Realizing the Promise of Biologics
Resources

Bio-Rad's Drug Discovery and Development Workflow Solutions
(PDF 2.2 MB)

BioLogics Workflow Solutions
(PDF 3 MB)

Changing the Treatment Landscape with the Development of Biologics
(PDF 245 KB)
