Hercules, CA — August 14, 2014 — Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb) today announced the launch of new rapid cell lysis kits that allow researchers to obtain reverse transcription quantitative PCR (RT-qPCR) data directly from cultured cells without the need for a separate RNA purification step. Bio-Rad’s SingleShot™ family of cell lysis RT-qPCR kits provide high-quality gene expression results in less than two hours.
Available column isolation methods for purifying RNA are time-consuming and laborious while other methods that enable RT-qPCR directly from cell lysates can damage the RNA and result in poor genomic DNA clearance. However, Bio-Rad’s SingleShot Kits eliminate those challenges and offer superior reproducibility and accuracy of gene expression results. In addition, minimal setup and pipetting steps create an automation-friendly workflow and, unlike other similar methods, Bio-Rad’s kits do not require an additional pipetting step to stop the cell lysis reaction.
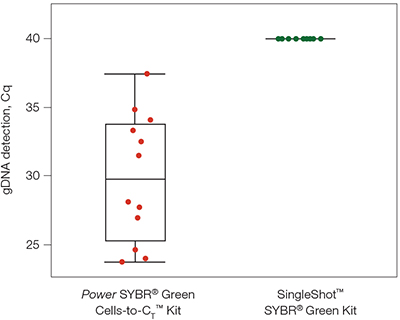
The SingleShot Kit removed genomic DNA (Cq > 40) while another available kit resulted in poor genomic DNA clearance.
“The SingleShot Kits are ideally suited for high-throughput laboratories with large-volume workloads and for researchers who are faced with a limited number of cells and require extreme accuracy in each analysis,” said Paul Streng, senior product manager in the Gene Expression Division of Bio-Rad’s Life Science Group.
Bio-Rad’s SingleShot Kits are the only available kits that include an RNA control template and qPCR assay to help researchers determine optimal cell number and lysate inputs for their RT-qPCR reactions.
SingleShot Kits are validated for use with a wide variety of adherent and suspension cell lines and are offered in multiple formats, including one-step RT-qPCR and two-step RT-qPCR kits that are compatible with either SYBR® Green or probe-based assays. SingleShot Kits are available as a stand-alone cell lysis kit. All SingleShot Kits are validated for use with PrimePCR™ Assays and Panels (PrimePCR Assays and Panels are available in the U.S., Canada, Australia, New Zealand, Singapore, and E.U. only).For more information on Bio-Rad’s RT-qPCR products, please visit www.bio-rad.com/SingleShotpr.
About Bio-Rad
Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb) develops, manufactures, and markets a broad range of innovative products and solutions for the life science research and clinical diagnostic markets. The company is renowned for its commitment to quality and customer service among university and research institutions, hospitals, public health and commercial laboratories, as well as the biotechnology, pharmaceutical, and food safety industries. Founded in 1952, Bio-Rad is based in Hercules, California, and serves more than 100,000 research and healthcare industry customers through its global network of operations. The company employs approximately 7,750 people worldwide and had revenues exceeding $2.1 billion in 2013. For more information, visit our website at www.bio-rad.com.
This release contains certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements generally can be identified by the use of forward-looking terminology such as, “believe,” “expect,” “may,” “will,” “intend,” “estimate,” “continue,” or similar expressions or the negative of those terms or expressions. Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. For further information regarding the Company's risks and uncertainties, please refer to the “Risk Factors” in the Company’s public reports filed with the Securities and Exchange Commission, including the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. The Company cautions you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. Bio-Rad Laboratories, Inc., disclaims any obligation to update these forward-looking statements.
For more information contact:
Richard Kurtz
Bio-Rad
510-741-5638
Richard_Kurtz@bio-rad.com
Chempetitive Group
312-997-2436 x112
kli@chempetitive.com
