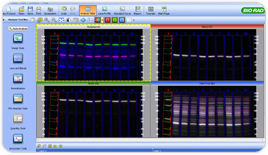
Software Provides Alternative to Potentially Misleading Housekeeping Proteins
Hercules, CA — September 5, 2012 — Bio-Rad Laboratories, Inc. announced today that it has introduced the new Image Lab 4.1 software for use with the ChemiDoc™ MP imaging system. The new software automates multiplex analysis, providing a faster, more accurate alternative for quantifying protein expression levels via western blotting.
Proving that quantitative differences of a target protein are a result of biological differences and not experimental variability (such as unequal protein loading) is critical when conducting western blotting experiments. Typically, scientists comparing gene expression levels use a housekeeping protein (HKP) as a loading control with the assumption that HKP expression does not change based on treatment type or sample origin. However, both of these conditions can alter HKP expression, undermining its value as a normalization control.
Total protein normalization has been shown to be more stable than HKP normalization regardless of conditions or sample type (ref DOI 10.1002/pmic.200400941). Also total protein normalization saves time by eliminating the need for multiple probing steps or optimizing a multiplex blot, both of which are often required to normalize with housekeeping proteins.
Normalizing total proteins via stain-free detection, a capability provided only by Bio-Rad‘s ChemiDoc MP imaging system, offers advantages including speed, increased linearity, and wider dynamic range, over other total protein detection methods such as SYPRO Ruby and Ponceau S.
Image Lab 4.1 automates total protein normalization of multiplex fluorescent western blots by extracting image data directly from the ChemiDoc MP imager. Traditional image analysis methods require researchers to extract data into a spreadsheet program and manually manipulate data to produce a result. With Image Lab 4.1 software, however, researchers can deliver normalized intensities automatically by selecting detection and normalization channels, then clicking the Normalization button. Normalization is available with chemiluminescent blots as well as multiplex fluorescent blots.
Stain-free total protein normalization is part of Bio-Rad’s new V3 Western Workflow, which speeds the western blotting process and provides researchers with the reassurance that their immunoblotting process is working as expected. Using stain-free detection, scientists can visualize protein separation without staining, verify protein transfer to the membrane, and validate the results by total protein normalization.
For product details or to order the ImageLab 4.1 software, visit www.bio-rad.com/imagelabsoftware.
About Bio-Rad
Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb) has remained at the center of scientific discovery for more than 50 years, manufacturing and distributing a broad range of products for the life science research and clinical diagnostic markets. The company is renowned worldwide among hospitals, universities, major research institutions, as well as biotechnology and pharmaceutical companies for its commitment to quality and customer service. Founded in 1952, Bio-Rad is headquartered in Hercules, California, and serves more than 100,000 research and industry customers worldwide through its global network of operations. The company employs over 7,100 people globally and had revenues exceeding $2 billion in 2011. For more information, visit www.bio-rad.com.
This release contains certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements generally can be identified by the use of forward-looking terminology such as, “believe,” “expect,” “may,” “will,” “intend,” “estimate,” “continue,” or similar expressions or the negative of those terms or expressions. Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. For further information regarding the Company's risks and uncertainties, please refer to the “Risk Factors” in the Company’s public reports filed with the Securities and Exchange Commission, including the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. The Company cautions you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. Bio-Rad Laboratories, Inc., disclaims any obligation to update these forward-looking statements.
For more information contact:
Ryan Short
Bio-Rad Laboratories, Inc.
800-876-3425
Ryan_Short@bio-rad.com
Ken Li
Chempetitive Group
312-997-2436 x 109
kli@chempetitive.com
